Publication
Article
Enasidenib Plus Venetoclax Yields High Response Rates Targeting IDH2 Mutations in R/R AML
Author(s):
The combination of the first-in-class inhibitor enasidenib plus venetoclax has generated excitement targeting IDH2 mutations in relapsed/refractory AML.
Steven Chan, MD, PhD

Data revealing encouraging efficacy and a well-tolerated safety profile of the first-in-class inhibitor enasidenib (Idhifa) plus venetoclax (Venclexta) have generated excitement among investigators in targeting IDH2 mutations for patients with relapsed or refractory acute myeloid leukemia (AML).
“The treatment of patients with relapsed/refractory AML remains challenging. The combination of enasidenib and venetoclax represents a potential option for the group of patients with AML [and] IDH2 mutations,” Steven Chan, MD, PhD, senior scientist at Princess Margaret Cancer Centre in Toronto, Ontario, said in a statement to OncologyLive. “For those who are eligible for an allogeneic stem cell transplant, this treatment may be an effective and lower-intensity option to achieve a remission and bridge to transplant.”
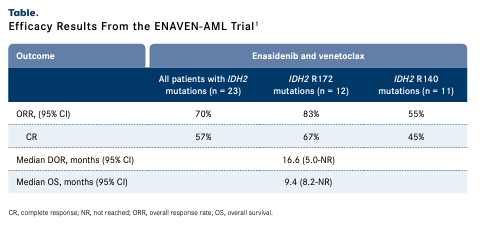
Findings from the phase 1b/2 ENAVEN-AML study (NCT04092179) revealed that the overall response rate (ORR) was 70% for patients with IDH2-mutant AML treated with enasidenib and venetoclax (n = 23), which included a 57% complete response (CR) rate (TABLE 1).1 The median duration of response was 16.6 months (95% CI, 5.0-not reached [NR]) and 6 patients remain on the study. Three patients proceeded to hematopoietic stem cell transplantation following CR.1
Efficacy Results From the ENAVEN-AML Trial1
Targeting IDH2 in AML
IDH2 mutations are prevalent in approximately 8% to 12% of patients with AML and are more frequent in those with natural-killer AML, occurring at a rate of 19%.2 Research has not clearly shown what the impact of IDH mutations on outcomes is, although some findings have indicated there may be a favorable prognosis with IDH2 mutations in the setting of standard intensive chemotherapy.3
Enasidenib has held an FDA indication since August 2017 as monotherapy for the treatment of IDH2-mutant relapsed or refractory AML.4 National Comprehensive Cancer Network guidelines, which cite enasidenib as a treatment for patients with relapsed/refractory AML and an IDH2 mutation, note that venetoclax may also be used with hypomethylating agents (HMAs) or low-dose cytarabine; low-intensity therapy with HMAs is also recommended.2 However, ivosidenib (Tibsovo) is only recommended as an option for patients with IDH1-mutated disease.
In addition to occurring in AML, IDH2 mutations as well as IDH1 mutations, are present in most low-grade diffuse gliomas.5 The mutations result in overproduction of R-2-hydroxyglutarate, epigenetic dysregulation, impaired cellular differentiation, and an immunosuppressive tumor microenvironment.
Combining IDH2 and BCL2 Inhibitors
In the open-label ENAVEN-AML study, response rates varied for patients with IDH2 R172 mutations (n = 12) compared with those with IDH2 R140 mutations (n = 11).1 Patients with IDH2 R172 mutations achieved an ORR of 83% comprised of a 67% CR rate, and those with IDH2 R140 mutations experienced an ORR of 55%, which included a 45% CR rate. Additional data revealed that the median overall survival (OS) was 9.4 months (95% CI, 8.2-NR) and the 18-month OS rate was 42% (95% CI, 27%-66%) among all patients with IDH2-mutated disease.
“In the ENAVEN trial, we found that the all-oral combination of enasidenib and venetoclax was associated with a high response rate in patients with relapsed/refractory IDH2-mutated AML, especially in those with a mutation at IDH2 R172,” Chan said. “The combination treatment was also well tolerated. Further studies comparing this combination regimen with other treatments in the frontline and relapsed/refractory settings are warranted.”
Following the phase 1b portion of the trial, the recommended phase 2 dose was determined to be 100 mg daily of enasidenib and 400 mg daily of venetoclax, each given on days 1 to 28 of every cycle.1
“The drugs in this combination are administered orally and once a day,” Chan noted. “The ease of administration of this regimen is advantageous over other combinations that require subcutaneous or intravenous injections.”
In the safety population (n = 27), hematological treatment-emergent adverse effects (TEAEs) that occurred at grades 1 to 2 and grades 3 to 5 included thrombocytopenia (4% and 26%, respectively), anemia (4% and 19%), neutropenia (0% and 15%), and leukocytosis (0% and 8%). Grade 1 to 2 nonhematologic TEAEs included nausea (41%), diarrhea (37%), hyperbilirubinemia (33%), constipation (30%), liver function test abnormalities (26%), electrolyte or metabolic abnormalities (22%), pneumonia (19%), and intracranial bleeding (4%). Grade 3 to 5 TEAEs included neutropenic fever (41%), pneumonia (22%), hyperbilirubinemia (15%), electrolyte or metabolic abnormalities (11%), liver function test abnormalities (7%), and diarrhea (4%) (TABLE 2).1
Safety Findings From the ENAVEN-AML Trial
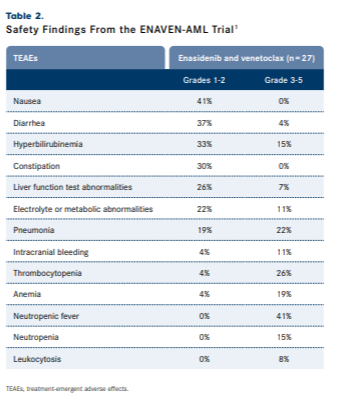
Severe AEs occurred in 30% of patients and included infections or neutropenic fever (26%) and small bowel obstruction (4%). Dose interruptions (56%); dose modifications (15%); and treatment discontinuation (78%) due to allogeneic stem cell transplant (15%) and progressive disease, relapse, or death (63%) were also recorded. Investigators noted that there was no significant myelosuppression observed and the infection rate was as expected for relapsed or refractory AML.1
“Overall, the safety profile of the combination regimen was similar to the ones associated with each drug alone. We did not observe any unexpected toxicities. Treatment with IDH inhibitors including enasidenib has been shown to cause differentiation syndrome. In our study cohort, differentiation syndrome was not observed. The addition of venetoclax to enasidenib may decrease the risk of developing this condition. However, a larger study is required to confirm this hypothesis,” Chan explained.
An IDH2 and IDH1 Inhibitor Makes Waves
The oral inhibitor vorasidenib (AG-881), which was designed for brain penetrance, significantly improved PFS at a preplanned interim analysis of the global phase 3 INDIGO trial (NCT04164901) in patients with IDH1- and IDH2-mutated residual or recurrent grade 2 glioma, meeting the trial’s primary end point.5 Patients who received the agent (n = 168) achieved a median PFS of 27.7 months (95% CI, 17.0-not estimable [NE]) vs 11.1 months (95% CI, 11.0-13.7) for patients who received placebo (n = 163; HR, 0.39; 95% CI, 0.27-0.56; 1-sided P = .000000067). All subgroups examined also experienced a benefit with vorasidenib.
Time to next intervention (TTNI) was also significantly improved with vorasidenib; the median TTNI was NR in the investigative arm vs 17.8 months (95% CI, 15.0-NE) in the placebo arm (HR, 0.26; 95% CI, 0.15-0.43; 1-sided P = .000000019); 24-month rates were 83.4% compared with 27.0%, respectively.5
The agent received fast track designation from the FDA in March 2023.6 Safety data presented with the preplanned interim efficacy analysis at the 2023 American Society of Clinical Oncology Annual Meeting revealed low discontinuation rates due to TEAEs at rates of 3.6% for vorasidenib vs 1.2% for placebo; grade 3 or higher AEs occurred at rates of 22.8% vs 13.5%, respectively.5 Treatment interruption (29.9% vs 22.7%) and dose reduction (10.8% vs 3.1%) due to AEs occurred in the vorasidenib and placebo arms, respectively, as well; no fatal TEAEs were observed.
Investigations Underway in Relapsed/Refractory Disease
A phase 2 study (NCT03683433) at The University of Texas MD Anderson Cancer Center in Houston is enrolling patients with recurrent or refractory IDH2-mutated AML to receive enasidenib in combination with azacitidine (Vidaza).7 Additionally, another phase 2 clinical trial (NCT04203316) is enrolling patients at least 24 months old but younger than 21 years old with relapsed or refractory IDH2-mutated AML to receive enasidenib monotherapy.8
References
- Richard-Carpentier G, Gupta G, Cameron C, et al. Final results of the phase Ib/II study evaluating enasidenib in combination with venetoclax in patients with IDH2-mutated relapsed/refractory myeloid malignancies. Blood. 2023;142(suppl 1):159. doi:10.1182/blood-2023-188341
- NCCN. Clinical Practice Guidelines in Oncology. Acute myeloid leukemia, version 6.2023. Accessed January 15, 2024. bit.ly/47E6Zo9
- Issa GC, DiNardo CD. Acute myeloid leukemia with IDH1 and IDH2 mutations: 2021 treatment algorithm. Blood Cancer J. 2021;11(6):107. doi:10.1038/s41408-021-00497-1
- FDA granted regular approval to enasidenib for the treatment of relapsed or refractory AML. FDA. August 1, 2017. Accessed January 15, 2024. bit.ly/3O3G2mM
- Mellinghoff IK, Van Den Bent MJ, Blumenthal DT, et al. INDIGO: a global, randomized, double-blinded, phase 3 study of vorasidenib versus placebo in patients with residual or recurrent grade 2 glioma with an IDH1/2 mutation. J Clin Oncol. 2023;41(suppl 17):LBA1. doi:10.1200/JCO.2023.41.17_suppl.LBA1
- Servier’s pivotal phase 3 INDIGO trial investigating vorasidenib in IDH-mutant low-grade glioma meets primary endpoint of progression-free survival (PFS) and key secondary endpoint of time to next intervention (TTNI). News release. Servier. March 14, 2023. Accessed January 15, 2024. https://prn.to/3U1mx25
- Enasidenib and azacitidine in treating patients with recurrent or refractory acute myeloid leukemia and IDH2 gene mutation. ClinicalTrials.gov. Updated August 18, 2023. Accessed January 15, 2024. bit.ly/3SjdXKQ
- Enasidenib for the treatment of relapsed or refractory acute myeloid leukemia patients with an IDH2 mutation. ClinicalTrials.gov. Updated December 11, 2023. Accessed January 15, 2024. bit.ly/3tXdmoL









