Article
FDA-Approved Adjuvant Treatment for Patients with High-Risk Urothelial Carcinoma
Sponsored by Bristol Myers Squibb
Until recently, the treatment landscape for urothelial carcinoma (UC) had remained unchanged for many years, and while this type of cancer can be diagnosed early, the rates of recurrence and disease progression can be high.1,2 Neoadjuvant chemotherapy and radical resection are the standards of care for muscle-invasive urothelial carcinoma (MIUC), with the latter typically performed with curative intent.3,4 However, the risk of recurrence may remain high for some patients despite undergoing major, life-changing surgery.5
Because of the complexity of this disease, patients diagnosed with UC are typically treated by a multidisciplinary team comprised of both a urologist and oncologist.6 Urologists are often patients’ first point of contact - providing their cancer diagnosis, introducing the initial treatment plan, and remaining involved throughout the journey.
Elan Diamond, MD

“Typically, we’re brought in to consult with urologists about their patients prior to radical resection to assess whether a patient may benefit from neoadjuvant or adjuvant treatment, which historically has meant chemotherapy or radiation,” said Elan Diamond, MD, medical director of Urologic Oncology, New Jersey Urology.7 “Since the treatment process is rarely linear, initial patient treatment conversations have primarily focused on the short term.”
Fortunately for these patients, recent scientific developments have led to a treatment option approved by the U.S. Food and Drug Administration (FDA) to help prevent recurrence for patients who are at high risk.
ADVANCES WITH IMMUNOTHERAPY FOR UC PATIENTS
In August 2021, the U.S. FDA approved Opdivo® (nivolumab) for the adjuvant treatment of adult patients with UC who are at high risk of recurrence after undergoing radical resection, regardless of prior neoadjuvant chemotherapy, nodal involvement or PD-L1 status.8 The approval, which was supported by data from the Phase 3 CheckMate -274 clinical trial, marked the first and only FDA-approved adjuvant treatment option to treat adult patients with UC who are at high risk of recurrence after undergoing radical resection.
Rian J. Dickstein, MD

“Our role as urologists enables us to have important early conversations with these patients soon after their cancer diagnosis about potential treatment options, and includes setting expectations that the approach to treating their disease may evolve over the course of treatment,” said Rian J. Dickstein, MD, FACS, director, Bladder Cancer Program, Chesapeake Urology, and chief of urology, University of Maryland Baltimore Washington Medical Center.
“In my experience, the established relationship between urologists and their patients creates an opportunity to introduce the concept of adjuvant immunotherapy treatment before patients are referred to an oncologist,” explained Dr. Diamond. “This is beneficial because discussions around treatment can be overwhelming, and therefore it’s helpful when patients aren’t surprised to learn additional treatment may be recommended to help prevent recurrence after undergoing surgery.”
WARNINGS AND PRECAUTIONS
Opdivo is associated with the following Warnings & Precautions: severe and fatal immune-mediated adverse reactions including pneumonitis, colitis, hepatitis and hepatotoxicity, endocrinopathies, dermatologic adverse reactions, nephritis with renal dysfunction, other immune-mediated adverse reactions; infusion-related reactions; complications of allogeneic hematopoietic stem cell transplantation (HSCT); embryo-fetal toxicity; and increased mortality in patients with multiple myeloma when Opdivo is added to a thalidomide analogue and dexamethasone, which is not recommended outside of controlled clinical trials.8 Please see Important Safety Information below.
CHECKMATE -274 METHODOLOGY AND DATA
CheckMate -274 was a randomized, double-blind, placebo-controlled, multi-center trial evaluating Opdivo as an adjuvant treatment in patients who had undergone radical resection of UC originating in the bladder or upper urinary tract and were at high risk of recurrence.8 The UC pathologic staging criteria that defines high-risk patients was ypT2-ypT4a or ypN+ for patients who received neoadjuvant cisplatin chemotherapy or pT3-pT4a or pN+ for patients who did not receive neoadjuvant cisplatin, and who also either were ineligible for or refused adjuvant cisplatin chemotherapy.8
Patients were randomized (n=353 and n=356 to the Opdivo and placebo arms, respectively) to receive Opdivo 240 mg or placebo by intravenous infusion over 30 minutes every two weeks until recurrence or unacceptable toxicity for a maximum treatment duration of one year.8 Eligible patients were randomized in a 1:1 ratio to Opdivo or placebo and were stratified by pathologic nodal status (N+ vs. N0/x with <10 nodes removed vs. N0 with ≥10 nodes removed), tumor cells expressing PD-L1 (≥1% vs. <1%/indeterminate as determined by the central lab using the PD-L1 IHC 28-8 pharmDx assay), and use of neoadjuvant cisplatin (yes vs. no).8
The major efficacy outcome measures were investigator-assessed disease-free survival (DFS) in all randomized patients and in patients with tumors expressing PD-L1 ≥1%. DFS was defined as time to first recurrence (local urothelial tract, local non-urothelial tract or distant metastasis), or death.8 Additional efficacy outcome measures included overall survival.8 The FDA-approved dosing for Opdivo is 240 mg every two weeks (30-minute intravenous infusion) or 480 mg every four weeks (30-minute intravenous infusion) until disease recurrence or unacceptable toxicity for up to one year.8
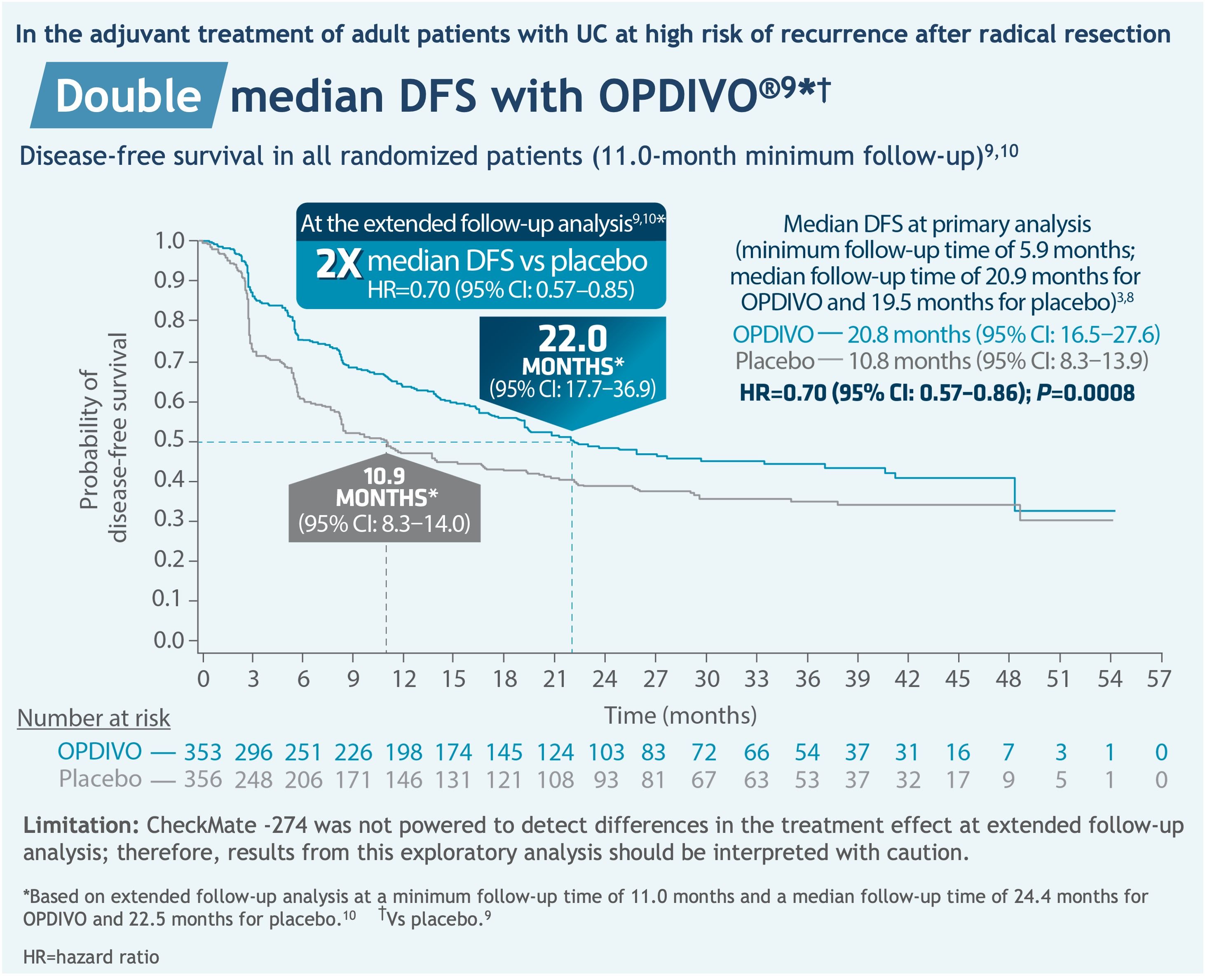
In the primary analysis, median DFS in patients with PD-L1 ≥1% (minimum follow-up time of 6.3 months; median follow-up time of 22.1 months for Opdivo and 18.7 months for placebo) was not reached for Opdivo (95% CI: 21.2-not estimable (NE)) and 8.4 months for placebo (95% CI: 5.6-21.2), with a hazard ratio (HR) of 0.55 (95% CI: 0.39-0.77; P=0.0005).8,11
In an extended follow-up analysis, median DFS in patients with PD-L1 ≥1% (minimum follow-up time of 11.4 months; median follow-up time of 25.5 months for Opdivo and 22.4 months for placebo) was not reached for Opdivo (95% CI: 22.1-NE), 8.4 months for placebo (95% CI: 5.6-20.0), with a HR of 0.53 (95% CI: 0.38-0.75).10
The most common (≥20%) adverse reactions were rash (36%), fatigue (36%), diarrhea (30%), pruritus (30%), musculoskeletal pain (28%), and urinary tract infection (22%). The most frequent (≥2%) serious adverse reaction in patients receiving Opdivo was urinary tract infection. Fatal adverse reactions occurred in 1% of patients and included pneumonitis (0.6%).8
“The findings from CheckMate -274 showed a reduction in the risk of disease recurrence or death with Opdivo for certain high-risk patients with UC by 30% compared to placebo,” said Dr. Diamond.8 “These data are meaningful, and the introduction of adjuvant immunotherapy provides physicians an additional treatment modality to consider for their patients facing a difficult-to-treat disease that has seen limited scientific progress in recent years.”
In exploratory subgroup analyses in patients with upper tract UC (n=149), no improvement in DFS was observed in the Opdivo arm compared to the placebo arm.8 The unstratified DFS HR estimate was 1.15 (95% CI: 0.74-1.80).8 In an exploratory subgroup analysis in patients with PD-L1 expression of <1% (n=414), the unstratified DFS HR estimate was 0.83 (95% CI: 0.64-1.08).8 Overall survival data is immature with 33% of deaths in the overall randomized population.8 In the upper tract UC subpopulation, 37 deaths occurred (20 in the Opdivo arm, 17 in the placebo arm).8
IN SUMMARY
In an area of high unmet need, immunotherapy for the adjuvant treatment of UC for high-risk patients in this setting is a welcomed advancement. Opdivo is the first and only FDA-approved option for the adjuvant treatment of UC in patients regardless of prior neoadjuvant chemotherapy, nodal involvement, or PD-L1 status.8
“Pairing surgery with adjuvant immunotherapy may help improve the chances of reducing disease recurrence for certain high-risk UC patients,” said Dr. Dickstein.8
To learn more about Opdivo, please visit www.OpdivoHCP.com.
INDICATION
OPDIVO® (nivolumab), as a single agent, is indicated for the adjuvant treatment of adult patients with urothelial carcinoma (UC) who are at high risk of recurrence after undergoing radical resection of UC.
IMPORTANT SAFETY INFORMATION
Severe and Fatal Immune-Mediated Adverse Reactions
Immune-mediated adverse reactions listed herein may not include all possible severe and fatal immune- mediated adverse reactions.
Immune-mediated adverse reactions, which may be severe or fatal, can occur in any organ system or tissue. While immune-mediated adverse reactions usually manifest during treatment, they can also occur after discontinuation of OPDIVO. Early identification and management are essential to ensure safe use of OPDIVO. Monitor for signs and symptoms that may be clinical manifestations of underlying immune-mediated adverse reactions. Evaluate clinical chemistries including liver enzymes, creatinine, and thyroid function at baseline and periodically during treatment with OPDIVO. In cases of suspected immune-mediated adverse reactions, initiate appropriate workup to exclude alternative etiologies, including infection. Institute medical management promptly, including specialty consultation as appropriate.
Withhold or permanently discontinue OPDIVO depending on severity (please see section 2 Dosage and Administration in the accompanying Full Prescribing Information). In general, if OPDIVO interruption or discontinuation is required, administer systemic corticosteroid therapy (1 to 2 mg/kg/day prednisone or equivalent) until improvement to Grade 1 or less. Upon improvement to Grade 1 or less, initiate corticosteroid taper and continue to taper over at least 1 month. Consider administration of other systemic immunosuppressants in patients whose immune-mediated adverse reactions are not controlled with corticosteroid therapy. Toxicity management guidelines for adverse reactions that do not necessarily require systemic steroids (e.g., endocrinopathies and dermatologic reactions) are discussed below.
Immune-Mediated Pneumonitis
OPDIVO can cause immune-mediated pneumonitis. The incidence of pneumonitis is higher in patients who have received prior thoracic radiation. In patients receiving OPDIVO monotherapy, immune-mediated pneumonitis occurred in 3.1% (61/1994) of patients, including Grade 4 (<0.1%), Grade 3 (0.9%), and Grade 2 (2.1%).
Immune-Mediated Colitis
OPDIVO can cause immune-mediated colitis. A common symptom included in the definition of colitis was diarrhea. Cytomegalovirus (CMV) infection/reactivation has been reported in patients with corticosteroid-refractory immune-mediated colitis. In cases of corticosteroid-refractory colitis, consider repeating infectious workup to exclude alternative etiologies. In patients receiving OPDIVO monotherapy, immune-mediated colitis occurred in 2.9% (58/1994) of patients, including Grade 3 (1.7%) and Grade 2 (1%).
Immune-Mediated Hepatitis and Hepatotoxicity
OPDIVO can cause immune-mediated hepatitis. In patients receiving OPDIVO monotherapy, immune-mediated hepatitis occurred in 1.8% (35/1994) of patients, including Grade 4 (0.2%), Grade 3 (1.3%), and Grade 2 (0.4%).
Immune-Mediated Endocrinopathies
OPDIVO can cause primary or secondary adrenal insufficiency, immune-mediated hypophysitis, immune-mediated thyroid disorders, and Type 1 diabetes mellitus, which can present with diabetic ketoacidosis. Withhold OPDIVO depending on severity (please see section 2 Dosage and Administration in the accompanying Full Prescribing Information). For Grade 2 or higher adrenal insufficiency, initiate symptomatic treatment, including hormone replacement as clinically indicated. Hypophysitis can present with acute symptoms associated with mass effect such as headache, photophobia, or visual field defects. Hypophysitis can cause hypopituitarism; initiate hormone replacement as clinically indicated. Thyroiditis can present with or without endocrinopathy. Hypothyroidism can follow hyperthyroidism; initiate hormone replacement or medical management as clinically indicated. Monitor patients for hyperglycemia or other signs and symptoms of diabetes; initiate treatment with insulin as clinically indicated.
In patients receiving OPDIVO monotherapy, adrenal insufficiency occurred in 1% (20/1994), including Grade 3 (0.4%) and Grade 2 (0.6%).
In patients receiving OPDIVO monotherapy, hypophysitis occurred in 0.6% (12/1994) of patients, including Grade 3 (0.2%) and Grade 2 (0.3%).
In patients receiving OPDIVO monotherapy, thyroiditis occurred in 0.6% (12/1994) of patients, including Grade 2 (0.2%).
In patients receiving OPDIVO monotherapy, hyperthyroidism occurred in 2.7% (54/1994) of patients, including Grade 3 (<0.1%) and Grade 2 (1.2%).
In patients receiving OPDIVO monotherapy, hypothyroidism occurred in 8% (163/1994) of patients, including Grade 3 (0.2%) and Grade 2 (4.8%).
In patients receiving OPDIVO monotherapy, diabetes occurred in 0.9% (17/1994) of patients, including Grade 3 (0.4%) and Grade 2 (0.3%), and 2 cases of diabetic ketoacidosis.
Immune-Mediated Nephritis with Renal Dysfunction
OPDIVO can cause immune-mediated nephritis. In patients receiving OPDIVO monotherapy, immune-mediated nephritis and renal dysfunction occurred in 1.2% (23/1994) of patients, including Grade 4 (<0.1%), Grade 3 (0.5%), and Grade 2 (0.6%).
Immune-Mediated Dermatologic Adverse Reactions
OPDIVO can cause immune-mediated rash or dermatitis. Exfoliative dermatitis, including Stevens-Johnson syndrome (SJS), toxic epidermal necrolysis (TEN), and drug rash with eosinophilia and systemic symptoms (DRESS) has occurred with PD-1/PD-L1 blocking antibodies. Topical emollients and/or topical corticosteroids may be adequate to treat mild to moderate nonexfoliative rashes.
Withhold or permanently discontinue OPDIVO depending on severity (please see section 2 Dosage and Administration in the accompanying Full Prescribing Information).
In patients receiving OPDIVO monotherapy, immune-mediated rash occurred in 9% (171/1994) of patients, including Grade 3 (1.1%) and Grade 2 (2.2%).
Other Immune-Mediated Adverse Reactions
The following clinically significant immune-mediated adverse reactions occurred at an incidence of <1% (unless otherwise noted) in patients who received OPDIVO monotherapy or were reported with the use of other PD-1/PD-L1 blocking antibodies. Severe or fatal cases have been reported for some of these adverse reactions: cardiac/vascular: myocarditis, pericarditis, vasculitis; nervous system: meningitis, encephalitis, myelitis and demyelination, myasthenic syndrome/myasthenia gravis (including exacerbation), Guillain-Barré syndrome, nerve paresis, autoimmune neuropathy; ocular: uveitis, iritis, and other ocular inflammatory toxicities can occur; gastrointestinal: pancreatitis to include increases in serum amylase and lipase levels, gastritis, duodenitis; musculoskeletal and connective tissue: myositis/polymyositis, rhabdomyolysis, and associated sequelae including renal failure, arthritis, polymyalgia rheumatica; endocrine: hypoparathyroidism; other (hematologic/immune): hemolytic anemia, aplastic anemia, hemophagocytic lymphohistiocytosis (HLH), systemic inflammatory response syndrome, histiocytic necrotizing lymphadenitis (Kikuchi lymphadenitis), sarcoidosis, immune thrombocytopenic purpura, solid organ transplant rejection.
Some ocular IMAR cases can be associated with retinal detachment. Various grades of visual impairment, including blindness, can occur. If uveitis occurs in combination with other immune-mediated adverse reactions, consider a Vogt-Koyanagi-Harada–like syndrome, which has been observed in patients receiving OPDIVO, as this may require treatment with systemic corticosteroids to reduce the risk of permanent vision loss.
Infusion-Related Reactions
OPDIVO can cause severe infusion-related reactions. Discontinue OPDIVO in patients with severe (Grade 3) or life-threatening (Grade 4) infusion-related reactions. Interrupt or slow the rate of infusion in patients with mild (Grade 1) or moderate (Grade 2) infusion-related reactions. In patients receiving OPDIVO monotherapy as a 60-minute infusion, infusion-related reactions occurred in 6.4% (127/1994) of patients. In a separate trial in which patients received OPDIVO monotherapy as a 60-minute infusion or a 30-minute infusion, infusion-related reactions occurred in 2.2% (8/368) and 2.7% (10/369) of patients, respectively. Additionally, 0.5% (2/368) and 1.4% (5/369) of patients, respectively, experienced adverse reactions within 48 hours of infusion that led to dose delay, permanent discontinuation or withholding of OPDIVO.
Complications of Allogeneic Hematopoietic Stem Cell Transplantation
Fatal and other serious complications can occur in patients who receive allogeneic hematopoietic stem cell transplantation (HSCT) before or after being treated with OPDIVO. Transplant-related complications include hyperacute graft-versus-host-disease (GVHD), acute GVHD, chronic GVHD, hepatic veno-occlusive disease (VOD) after reduced intensity conditioning, and steroid-requiring febrile syndrome (without an identified infectious cause). These complications may occur despite intervening therapy between OPDIVO and allogeneic HSCT.
Follow patients closely for evidence of transplant-related complications and intervene promptly. Consider the benefit versus risks of treatment with OPDIVO prior to or after an allogeneic HSCT.
Embryo-Fetal Toxicity
Based on its mechanism of action and findings from animal studies, OPDIVO can cause fetal harm when administered to a pregnant woman. Advise pregnant women of the potential risk to a fetus. Advise females of reproductive potential to use effective contraception during treatment with OPDIVO and for at least 5 months after the last dose.
Increased Mortality in Patients with Multiple Myeloma when OPDIVO is Added to a Thalidomide Analogue and Dexamethasone
In randomized clinical trials in patients with multiple myeloma, the addition of OPDIVO to a thalidomide analogue plus dexamethasone resulted in increased mortality. Treatment of patients with multiple myeloma with a PD-1 or PD-L1 blocking antibody in combination with a thalidomide analogue plus dexamethasone is not recommended outside of controlled clinical trials.
Lactation
There are no data on the presence of OPDIVO in human milk, the effects on the breastfed child, or the effects on milk production. Because of the potential for serious adverse reactions in breastfed children, advise women not to breastfeed during treatment and for 5 months after the last dose.
Serious Adverse Reactions
In Checkmate 274, serious adverse reactions occurred in 30% of patients receiving OPDIVO (n=351). The most frequent serious adverse reaction reported in ≥2% of patients receiving OPDIVO was urinary tract infection. Fatal adverse reactions occurred in 1% of patients; these included events of pneumonitis (0.6%).
Common Adverse Reactions
In Checkmate 274, the most common adverse reactions (≥20%) reported in patients receiving OPDIVO (n=351) were rash (36%), fatigue (36%), diarrhea (30%), pruritus (30%), musculoskeletal pain (28%), and urinary tract infection (22%).
Please see US Full Prescribing Information and Medication Guide for OPDIVO.
References
- American Cancer Society. Bladder Cancer Early Detection, Diagnosis, and Staging https://www.cancer.org/content/dam/CRC/PDF/Public/8559.00.pdf. Accessed October 2022.
- Chamie K, Litwin MS, Bassett JC, et al. Recurrence of high-risk bladder cancer: A population-based analysis. Cancer. 2013;119(17):3219-3227.
- Bajorin DF, Wities JA, Gschwend JE, et al. Adjuvant Nivolumab versus Placebo in Muscle-Invasive Urothelial Carcinoma. N Engl J Med. 2021;384:2102-2114.
- Aragon-Ching JB, Werntz RP, Zietman AL, Steinberg GD. Multidisciplinary Management of Muscle-Invasive Bladder Cancer: Current Challenges and Future Directions. Am Soc Clin Oncol Educ Book. 2018;38:307-318.
- Apolo AB, Msaouel P, Niglio S, et al. Evolving Role of Adjuvant Systemic Therapy for Kidney and Urothelial Cancers. Am Soc Clin Oncol Educ Book. 2022;42:1-16.
- American Cancer Society. Treating Bladder Cancer. https://www.cancer.org/cancer/bladder-cancer/treating.html. Accessed October 2022.
- American Cancer Society. If You Have Bladder Cancer. https://www.cancer.org/cancer/bladder-cancer/if-you-have-bladder-cancer.html. Accessed October 2022.
- Opdivo Prescribing Information. Opdivo U.S. Product Information. Last updated: 07/2022. Princeton, NJ: Bristol-Myers Squibb Company.
- Witjes JA, Bajorin DF, Galsky MD, et al. Results for patients with muscle-invasive bladder cancer in the CheckMate 274 trial. Poster presentation at ASCO 2022. Abstract 4585.
- Galsky MD, Witjes JA, Gschweend JE, et al. Disease-free survival with longer follow-up from the phase 3 CheckMate 274 trial of adjuvant nivolumab in patients who underwent surgery for high-risk muscle-invasive urothelial carcinoma. Oral presentation at the American Urological Association (AUA) Annual Meeting 2022. Abstract 22-3807.
- Data on File. NIVO 639. Princeton, NJ: Bristol-Myers Squibb Company; 2021.
© 2022 Bristol-Myers Squibb Company.
OPDIVO® and the related logos are registered trademarks of Bristol-Myers Squibb Company.
1506-US-2200592 12/22