Article
Cabozantinib (XL184) Demonstrates Antitumor Activity and Reduces or Eliminates Bone Metastases in Multiple Cancers
Author(s):
Cabozantinib (XL184), an investigational oral tyrosine kinase inhibitor, demonstrated antitumor activity in multiple types of advanced solid tumors.
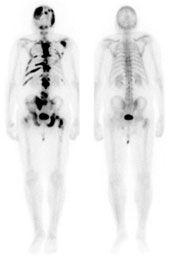
Before treatment (left) and after (right)
In a phase II randomized trial, cabozantinib (XL184), an investigational oral tyrosine kinase inhibitor, demonstrated antitumor activity in multiple types of advanced solid tumors. Prostate cancer, ovarian cancer, and hepatocellular carcinoma had the highest disease control rate, which combines partial response and stable disease. In an unexpected additional outcome, cabozantinib reduced or eliminated bone metastases in patients with breast and prostate cancers, as well as melanoma.
Cabozantinib inhibits the MET and VEGFR2 pathways, which contribute to the genesis and growth of tumor cells. The trial evaluated 398 patients with advanced, progressive solid tumors in 9 cancers with various levels of MET and VEGFR2 activity, including pancreatic cancer, castration-resistant prostate cancer (CRPC), hepatocellular carcinoma (HCC), gastric or gastroesophageal junction cancer, melanoma, small cell lung cancer (SCLC), ovarian cancer, breast cancer, and non—small cell lung cancer (NSCLC). Thirty-nine percent of patients had bone metastases.
The study was a randomized discontinuation trial, in which all patients initially received 100 mg of cabozantinib daily, and then treatment was either maintained or discontinued based on efficacy. At the 12-week follow-up point, patients with partial response remained on cabozantinib, subjects with stable disease were randomized to cabozantinib or placebo, and those with disease progression discontinued treatment.

Nicholas Vogelzang, MD
The overall response rate at 12 weeks was 9%. Specific response rates for individual tumor sites included ovarian (24%), HCC (14%), CRPC (5%), NSCLC (10%), breast (10%), SCLC (5%), and melanoma (5%). The highest disease control rates at follow-up were HCC (76%), CRPC (71%), ovarian cancer (58%), melanoma (45%), breast cancer (45%), and NSCLC (40%). Tumor shrinkage was observed in both internal organs and lymph nodes.
Bone scans also revealed that bone metastases decreased in 59 of 68 patients who had bone lesions. These results were confirmed through an independent review conducted by radiologists. The researchers had expected the tumor response based on preclinical studies of cabozantinib; however, they were surprised at the bone lesion activity, according to co-author Nicholas J. Vogelzang, MD, chair and medical director, developmental therapeutics committee and US Oncology research medical oncologist, Comprehensive Cancer Centers of Nevada in Las Vegas. The bone metastases improvement largely occurred in patients with CRPC, with complete or partial bone scan resolution in 86% of CRPC patients.
The bone metastases data for CRPC and the high tumor response rate for ovarian cancer led to the expansion of both tumors’ cohorts. The cohorts of the other tumor types were not expanded, but researchers are continuing to evaluate them.
The most common high-grade adverse events were fatigue, hand-foot syndrome, and hypertension at 9%, 8%, and 5%, respectively. Adverse reactions resulted in dose reductions in 41% of patients, and 12% of patients had to discontinue treatment. Cabozantinib is “not a benign drug, but it is clearly effective,” said Vogelzang.
Based on the study results, cabozantinib’s manufacturer, Exelixis, plans to commence a pivotal phase III trial in prostate cancer by the end of 2011. The company also intends to design additional phase III trials with other tumor types that will examine cabozantinib’s survival benefits and its use in combination with other treatments.
Exelixis is already conducting a phase III trial of cabozantinib in advanced medullary thyroid cancer (MTC). The FDA granted cabozantinib orphan drug status for MTC in January. The orphan designation provides marketing exclusivity and tax benefits to companies manufacturing treatments for rare diseases. Exelixis hopes to release the phase III results and potentially file a New Drug Application with the FDA for the MTC indication sometime this year.
Gordon MS, Vogelzang NJ, Schoffski P, et al. Cabozantinib (XL184) has activity in both soft tissue and bone: results of a phase II randomized discontinuation trial in patients with advanced solid tumors. J Clin Oncol. 2011. (suppl; abstract 3010)









.jpg?fit=crop&auto=format)