Publication
Article
B7-H4 Offers Potential Treatment Target in a Variety of Solid Tumors
Author(s):
The transmembrane protein B7-H4 has emerged as an interesting therapeutic target in multiple solid tumors, with investigators mostly focusing on the development of antibody-drug conjugates aimed at the pathway.
B7-H4 in Solid Tumors | Image Credit:
© Christoph Burgstedt - stock.adobe.com
The transmembrane protein B7-H4 has emerged as an interesting therapeutic target in multiple solid tumors, with investigators mostly focusing on the development of antibody-drug conjugates (ADCs) aimed at the pathway. With findings from multiple clinical trials showing promise in the space, investigators hope to progress B7-H4–directed agents to later-phase trials and ultimately add them to the treatment paradigm of some historically hard to treat tumor types.
“B7-H4 has regulatory activity being that it’s expressed by both tumor cells as well as myeloid cells that will infiltrate into the tumors,” Joseph R. Podojil, PhD, a research associate professor of microbiology-immunology at Northwestern University Feinberg School of Medicine in Chicago, Illinois, said in an interview with OncologyLive®. “As a consequence of its expression, it tends to induce a regulatory phenotype or the induction or expansion of regulatory T cells themselves within the [tumor] microenvironment.”
Although B7-H4 mRNA is widely expressed—having been found in tissues of the brain, heart, kidney, liver, lung, ovaries, and other locations—the expression of the B7-H4 protein on the surface of healthy cells is limited. However, overexpression of B7-H4 has been reported in many tumor types, including ovarian, lung, renal, breast, and gastric cancers. B7-H4 expression has been associated with increased tumor size, increased primary tumor classification, and/or diminished survival in these and other tumor types.1 Additionally, the protein has a role in tumorigenesis and tumor development via cell proliferation, invasion, metastasis, antiapoptosis, and other mechanisms.2
Preclinical Study Provides Proof of Concept to Target B7-H4
Although B7-H4–targeted therapeutics are still in relatively early stages of development, with no FDA-approved agents to date, findings from preclinical and early-phase studies have validated initial interest in the pathway. B7-H4–directed ADCs have displayed promise in a variety of tumor types, including breast, ovarian, and bladder cancers. Patients with these cancers often still have significant unmet needs for safe and effective targeted therapies, especially after relapsing on or becoming refractory to standard-of-care treatments.
In April 2020, Podojil et al published findings in Oncoimmunology demonstrating that blocking B7-H4 with an antibody led to an enhanced immune response in urothelial carcinoma. In preclinical models, treatment with the anti–B7-H4 antibody led to enhanced IFN-γ secretion by CD4/8-positive T cells in human monocyte and T-cell cocultures. Treatment with the anti–B7-H4 antibody of bladder cancer tumor models resulted in tumor shrinkage, elevated CD8-positive T-cell infiltration within the bladder, and a decrease in tumor-infiltrating T regulatory cells. Moreover, the combination of an anti–PD-1 antibody and the anti–B7-H4 antibody caused a significant reduction in tumor stage, size, and increased tumor necrosis.3
“We tested [B7-H4 inhibition] in a bladder cancer model using a carcinogen within the water,” Podojil said. “We saw good functionality of a blocking antibody. This antibody wasn’t functionalized [and] it wasn’t inducing antibody-dependent cellular cytotoxicity, but it inhibited the receptor-ligand interactions negative signals that B7-H4 would be inducing within the environment. It had a similar level of activity or greater [activity] than anti–PD-1 blockade, and in cotherapy we saw an additive effect of the 2.”
During the 2023 American Association for Cancer Research (AACR) Annual Meeting in Orlando, Florida, Gitto et al presented preclinical findings that established the expression of B7-H4 is maintained following resistance to standard-of-care therapy with platinum-based chemotherapy and/or PARP inhibitors in high-grade serous ovarian carcinoma (HGSOC) tumors. Additionally, they evaluated the preliminary efficacy of a novel B7-H4–directed ADC containing a pyrrolobenzodiazepine-dimer payload tesirine in preclinical models of breast and ovarian cancers.4
To conduct their study, investigators measured B7-H4 expression via quantitative flow cytometry and immunohistochemistry in patients with HGSOC at diagnosis and then after acquiring multidrug resistance. The transmembrane protein was found to be present in 92% of HGSOC tumors at diagnosis (n = 12). Recurrent matched samples showed that B7-H4 expression persisted after resistance to platinum-based chemotherapy and was present at similar levels to baseline at multiple metastatic sites following acquired multidrug resistance in 4 donors.
In terms of efficacy, treatment with the B7-H4–directed ADC led to target-specific growth inhibition in B7-H4–expressing ovarian and breast cancer cell lines (n = 10), with a median half-maximal inhibitory concentration of 48.3 pM (range, 6.18-273.9). In breast and ovarian patient-derived xenografts in vivo (n = 23), the ADC caused tumor regression at a rate of 61% at a 1-time dose of 0.3 mg/kg, with lower activity reported in models that were B7-H4 low or negative (P = .048). Moreover, in HGSOC patient-derived xenograft models (n = 3), treatment with the ADC once every 28 days continuously resulted in sustained partial (n = 3/16) and complete responses (CRs; n = 7/16) and extended survival (P < .004). Study authors concluded that their findings support B7-H4 as an attractive target for ADC therapy in patients with drug-resistant HGSOC.
“If you block [B7-H4], you’re taking away that inhibitory signal that effective CD8s and CD4s would receive within the local microenvironment such that you’re allowing for those cells to have antitumoral activity,” Podojil explained. “As far as its connection with other coinhibitory pathways, [such as] CTLA-4 and PD-1/L1, [B7-H4] is differentially expressed in comparison with those. This makes it an attractive cotherapy such that you could potentially use checkpoint inhibitors against those other pathways in combination with the therapeutic that would target B7-H4 itself. It also tends to be expressed by the tumors, so one can imagine not only using an antibody to inhibit its activity but using an antibody that has been functionalized [to produce] antitumoral activity.”
Early-Phase Study Findings Show Efficacy, Tolerability
Beyond preclinical study, findings from multiple first-in-human studies of novel B7-H4–directed ADCs were presented during the 2023 European Society for Medical Oncology Congress in Madrid, Spain, in October.
The novel B7-H4–directed ADC HS-20089 showed initial efficacy, with a tolerable safety profile, in patients with advanced solid tumors in a phase 1 trial (NCT05263479). HS-20089 is composed of a humanized anti–B7-H4 monoclonal antibody with a small-molecule toxin topoisomerase I inhibitor. In preclinical study findings, HS-20089 showed high affinity to B7-H4 and antitumor activity. In this first-in-human trial, patients with locally advanced or metastatic solid tumors, irrespective of B7-H4 status, received intravenous HS-20089 once every 3 weeks at doses ranging from 0.7 mg/kg to 7.2 mg/kg.5
At the June 30, 2023, data cutoff, patients with triple-negative breast cancer (TNBC) treated at any dose level (n = 28) experienced an objective response rate (ORR) of 28.6% (95% CI, 13.2%-48.7%) and a disease control rate (DCR) of 75.0% (95% CI, 55.1%-89.3%). Notably, among patients with TNBC who received a prior PARP inhibitor (n = 4), these rates at any dose level were 75.0% (95% CI, 19.4%-99.4%) and 100.0% (95% CI, 39.8%-100.0%), respectively. Among 3 patients with ovarian cancer, the ORR and DCR were 66.7% (95% CI, 9.4%-99.2%) and 100.0% (95% CI, 29.2%-100.0%), respectively, regardless of dose level.
In terms of safety, dose-limiting toxicities (DLTs) were observed in 1 patient at the 5.8-mg/kg dose level and 2 patients at 7.2 mg/kg. Any-grade treatment-emergent adverse effects (TEAEs) occurred in the overall population (n = 52) at a rate of 98.1% and no events leading to death or treatment discontinuation were reported.
The maximum tolerated dose of HS-20089 was determined to be 5.8 mg/kg. Investigators concluded their presentation by noting that the phase 1b expansion portion is ongoing, evaluating the agent at the 4.8-mg/kg and 5.8-mg/kg dose levels in TNBC, ovarian cancer, and other tumor types.
In another phase 1 trial (NCT05194072), the investigational vedotin ADC SGN-B7H4V is being examined in patients with relapsed/refractory solid tumors. The agent consists of aB7-H4–directed monoclonal antibody conjugated to monomethyl auristatin E.6
Part A of the study enrolled patients with locally advanced unresectable or metastatic solid tumors, regardless of B7-H4 expression level. Patients were treated with SGN-B7H4V at doses ranging from 0.75 mg/kg to 1.5 mg/kg on days 1 and 8 of each 21-day cycle (n = 41) or 1.25 mg/kg to 2.0 mg/kg on days 1 and 15 of each 28-day cycle (n = 45).
At the March 10, 2023, data cutoff, responses were observed in patients with breast (n = 7/28), ovarian (n = 4/20), lung (n = 1/6), and biliary tract (n = 2/11) cancers across dose levels. Notably, 1 patient in the endometrial cancer cohort (n = 16) achieved a CR.
Safety findings showed that any-grade TEAEs occurred at rates of 90.2% and 91.1% in the every-3-weeks and every-4-weeks dosing groups, respectively. Grade 3 or higher TEAEs (46.3% vs 40.0%) and TEAEs leading to dose reduction (17.1% vs 13.3%) occurred at both levels. No treatment-related deaths were reported. DLTs occurred in 4 patients in the every-3-weeks group and 3 patients in the every-4-weeks group. Study authors noted that patients continue to receive treatment on the every-4-weeks group dosing schedule and dose expansion is planned in select tumor types.
Additional Clinical Trials Look to Advance New ADCs
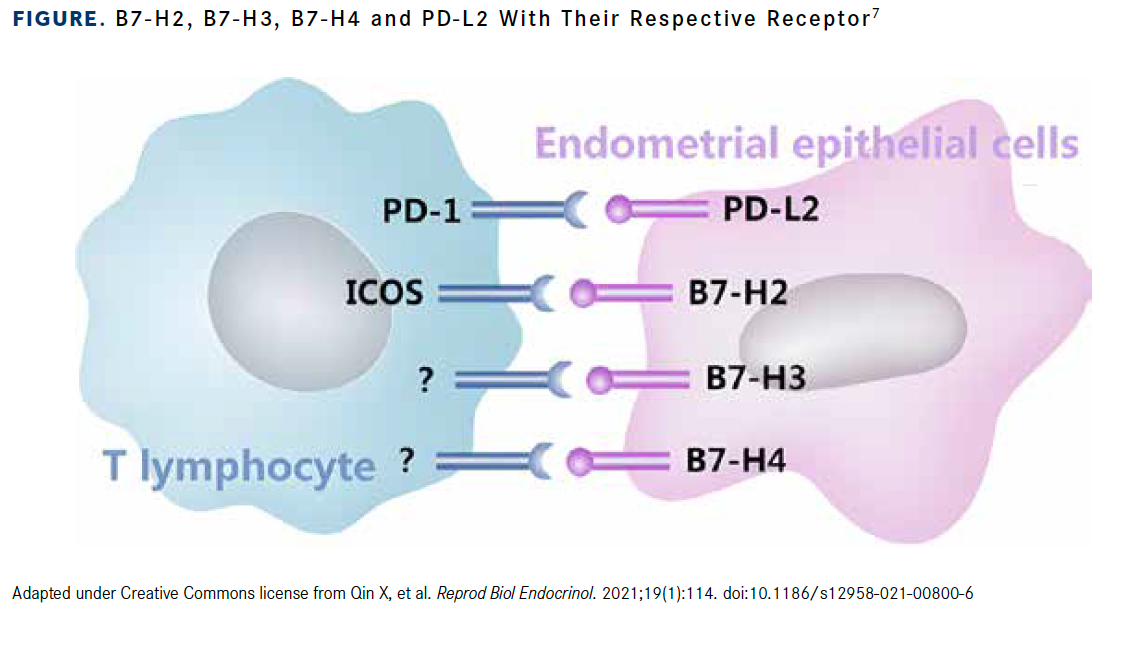
Building on the foundation laid out in preclinical and other early-phase studies, multiple B7-H4–directed ADCs are now being examined in clinical trials enrolling patients with a variety of tumor types (Figure).7 At the 2023 American Society of Clinical Oncology Annual Meeting in Chicago, Illinois, investigators presented study designs for multiple phase 1 clinical trials.
Figure. B7-H2, B7-H3, B7-H4 and PD-L2 With Their Respective Receptor7
In a phase 1/2a study (NCT05123482), investigators are examining the B7-H4–directed ADC AZD8205. The agent has shown the ability to induce cell death by intracellular delivery of its topoisomerase I inhibitor warhead and via the bystander killing effect.8
The first-in-human study is recruiting patients with locally advanced or recurrent cholangiocarcinoma, breast cancer, ovarian cancer, and endometrial cancer. Patients must be 18 years or older (≥20 years in Japan), have relapsed or metastatic disease after standard-of-care treatments, and have an ECOG performance status of 0 or 1. AZD8205 will be given via intravenous infusion to groups of 3 to 12 patients at 5 different dosing levels.
The primary end point is safety and tolerability. Secondary end points include ORR, DCR at 12 weeks, pharmacokinetics, and pharmacodynamics. The study is currently recruiting patients in the United States, Australia, Japan, and South Korea.
Finally, in a phase 1b trial (NCT05377996), investigators are evaluating the novel B7-H4–directed ADC XMT-1660. The agent uses its unique design to produce a controlled bystander effect in addition to inducing cell death via delivery of the auristatin F hydroxypropylamide payload. The first-in-human study is enrolling patients with breast, endometrial, and ovarian cancers after progression on standard-of-care therapies.9
The dose-expansion phase will enroll approximately 42 patients, with primary end points of determining the maximum tolerated dose, safety, and tolerability. The dose-expansion phase will divide patients into 3 potential cohorts: those with TNBC, endometrial or ovarian cancer, and hormone receptor–positive/HER2-negative breast cancer. Its primary end points are ORR and safety.
“Patients who receive therapies such as PD-1/L1 blockade experience increased survival rates. However, there are still patients who do not respond,” Podojil concluded. “Those with TNBC and small cell lung carcinoma are examples of patients who often do not fully respond to [available] checkpoint inhibitors, but these tumors have been shown to express B7-H4, making it a likely target to [develop] some therapeutics that would be able to help these patient populations.”
References
- Podojil JR, Miller SD. Potential targeting of B7-H4 for the treatment of cancer. Immunol Rev. 2017;276(1):40-51. doi:10.1111/imr.12530
- Wang JY, Wang WP. B7-H4, a promising target for immunotherapy. Cell Immunol. 2020;347:104008. doi:10.1016/j.cellimm.2019.104008
- Podojil JR, Glaser AP, Baker D, et al. Antibody targeting of B7-H4 enhances the immune response in urothelial carcinoma. Oncoimmunology. 2020;9(1):1744897. doi:10.1080/2162402X.2020.1744897
- Gitto SB, Whicker M, Davies G, et al. A B7-H4 targeting antibody-drug conjugate shows anti-tumor activity in PARPi and platinum resistant cancers with B7-H4 expression. Cancer Res. 2023;83(suppl 7):1133. doi:10.1158/1538-7445.AM2023-1133
- Wu J, Zhang J, Li H, et al. First-in-human/phase I trial of HS-20089, a B7-H4 ADC, in patients with advanced solid tumors. Ann Oncol. 2023;34(suppl 2):S336. doi:10.1016/j.annonc.2023.09.558
- Perez CA, Henry JT, Lakhani N, et al. First-in-human study of SGN-B7H4V, a B7-H4-directed vedotin ADC, in patients with advanced solid tumors: preliminary results of a phase I study (SGNB7H4V-001). Ann Oncol. 2023;34(suppl 2):S464-S465. doi:10.1016/j.annonc.2023.09.1846
- Qin X, Sun W, Wang C, et al. Mifepristone inhibited the expression of B7-H2, B7-H3, B7-H4 and PD-L2 in adenomyosis. Reprod Biol Endocrinol. 2021;19(1):114. doi:10.1186/s12958-021-00800-6
- Meric-Bernstam F, Oh DY, Naito Y, et al. First-in-human study of the B7-H4 antibody-drug conjugate (ADC) AZD8205 in patients with advanced/metastatic solid tumors. J Clin Oncol. 2022;40(suppl 16):TPS3153. doi:10.1200/JCO.2022.40.16_suppl.TPS3153
- Hamilton EP, Chaudhry A, Spira AI, et al. XMT-1660: a phase 1b trial of a B7-H4 targeted antibody drug conjugate (ADC) in breast, endometrial, and ovarian cancers. J Clin Oncol. 2023;41(suppl 16):TPS3154. doi:10.1200/JCO.2023.41.16_suppl.TPS3154