Article
Clinical Trials Propel New Standards of Care for Thyroid Cancer
Author(s):
Alan L. Ho, MD, PhD, discusses targeted therapies such as TKIs that have demonstrated promising efficacy in multiple patient populations as well as the agents lenvatinib and pembrolizumab.
Alan L. Ho, MD, PhD

Multidisciplinary approaches remain crucial as treatment options rely on expert insights to cover the wide array of thyroid cancer subgroups, according to Alan L. Ho, MD, PhD. The wider the expanse of input, the better chance of expanding and delivering clinical trials suited for patients with unmet needs.
“More than a decade ago there were few therapeutic options for these diseases,” Ho said. “We now have multiple targeted therapy options driven by targeted agents, which were studied in biologically rational clinical trials and targeted agents. Thyroid cancer, even more so than other diseases, has [been privy to] the fruits of the genomic era [and] advances in drug development, but more needs to be done to better understand the biology of the tumors and developing better therapies for patients.”
Ho, a medical oncologist and the Geoffrey Beene Junior Faculty Chair at Memorial Sloan Kettering Cancer Center in New York, New York, is the moderator of the Head, Neck, and Thyroid Cancer session. On Friday, November 11, he gave a talk titled “Therapeutics for Thyroid Cancer” during the 40th Annual Chemotherapy Foundation Symposium® (CFS®).
In an interview with OncLive® Ho discussed targeted therapies such as tyrosine kinase inhibitors (TKIs) that have demonstrated promising efficacy in multiple patient populations as well as the agents lenvatinib (Lenvima) and pembrolizumab (Keytruda).
What updates in the head and neck space were shared at CFS®?
[With the meeting,] the audience had the opportunity to get a handle on new standards of care and new developments in all different types of solid tumor malignancies outside of one’s specialty. In the head and neck session specifically, we had excellent speakers and talks about HPV-driven head and neck squamous cell carcinoma, which is an important topic right now given that [histology] represents most patients with head neck cancer in the US. We don’t tailor our therapies well enough to the HPV status of tumors as we can and there are exciting new areas which can [help] define how we should do that.
Another talk [highlighted the role of] immunotherapy in squamous cell carcinomas where there have been major advances and changes to the standard of care for those patients. Beyond my own specialty, it’s always educational to hear more about advances in diseases.
[In head and neck cancer] we reviewed many of the recent advances in developmental therapeutics for different types of thyroid cancer. It’s been an exciting time where we’ve [seen the] development of many targeted agents against specific genetic alterations in different thyroid cancer histology types. We reviewed the updated data [and how it affects] standards of care.
Some of the interesting biologic insights from these trials are that a few of the same regimens will have different efficacy profiles, whether [for] nonanaplastic or anaplastic thyroid cancers. [For example,] lenvatinib and pembrolizumab have been evaluated both in anaplastic and nonanaplastic populations. There’s some very promising response data that’s come out in trials enrolling patients with anaplastic thyroid cancer. Although toxicity, morbidity, and complications remain an issue that we must watch out for.
The landscape in nonanaplastic thyroid cancers is a little different, but there are interesting data regarding pembrolizumab’s role [and how it] may extend the benefit of lenvatinib in patients who already have grown resistant to it. There are different potential roles or clinical usage of these combinations depending upon what [type of] thyroid cancer a patient has.
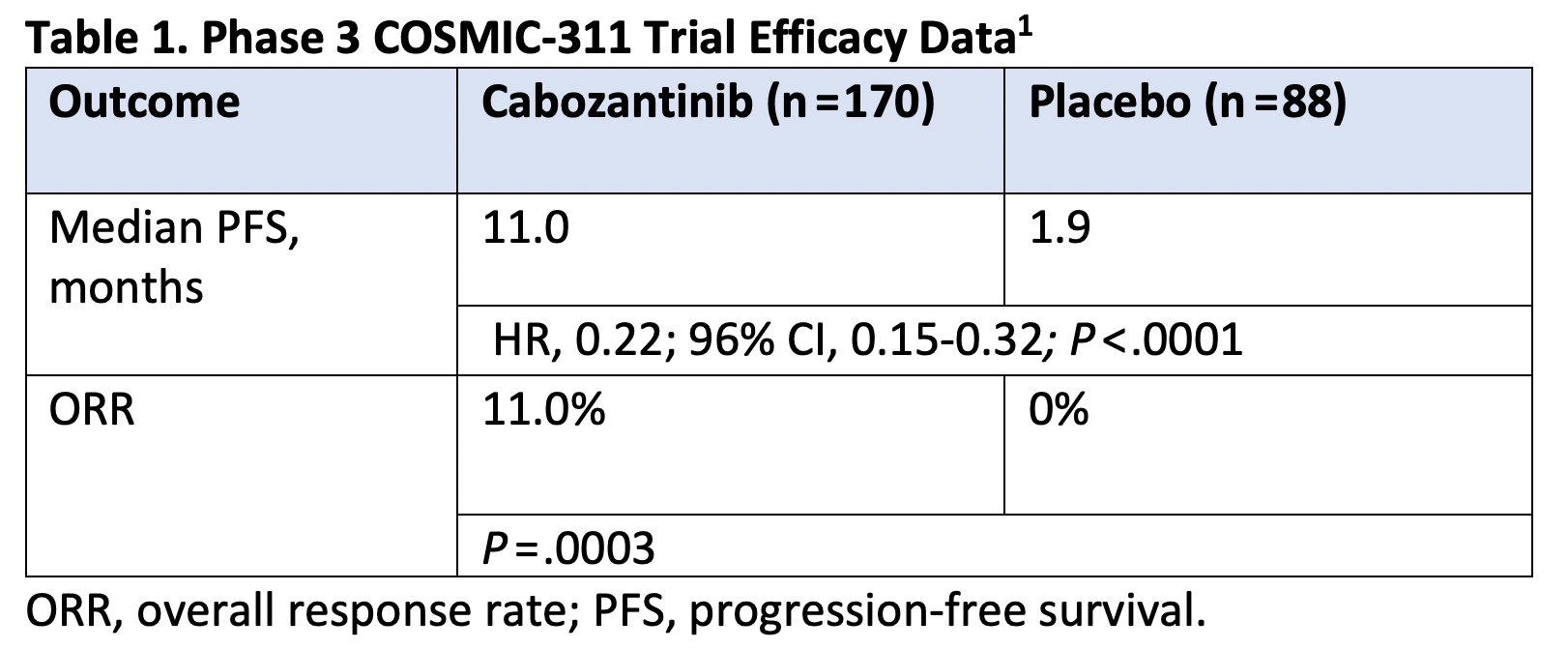
What did the COSMIC-311 trial (NCT03690388) show about the survival benefits of cabozantinib monotherapy in radioiodine-refractory, differentiated thyroid cancer? (Table 1)1
COSMIC-311 is the third phase 3 trial that has been formed for radioiodine-refractory thyroid cancer. This trial demonstrates for the first time the PFS [progression-free survival] benefit of cabozantinib (Cabometyx) over placebo in patients who have had previous TKI treatments, either 1 or 2 prior lines, and established cabozantinib’s efficacy in the setting.
Table 1. Phase 3 COSMIC-311 Trial Efficacy Data
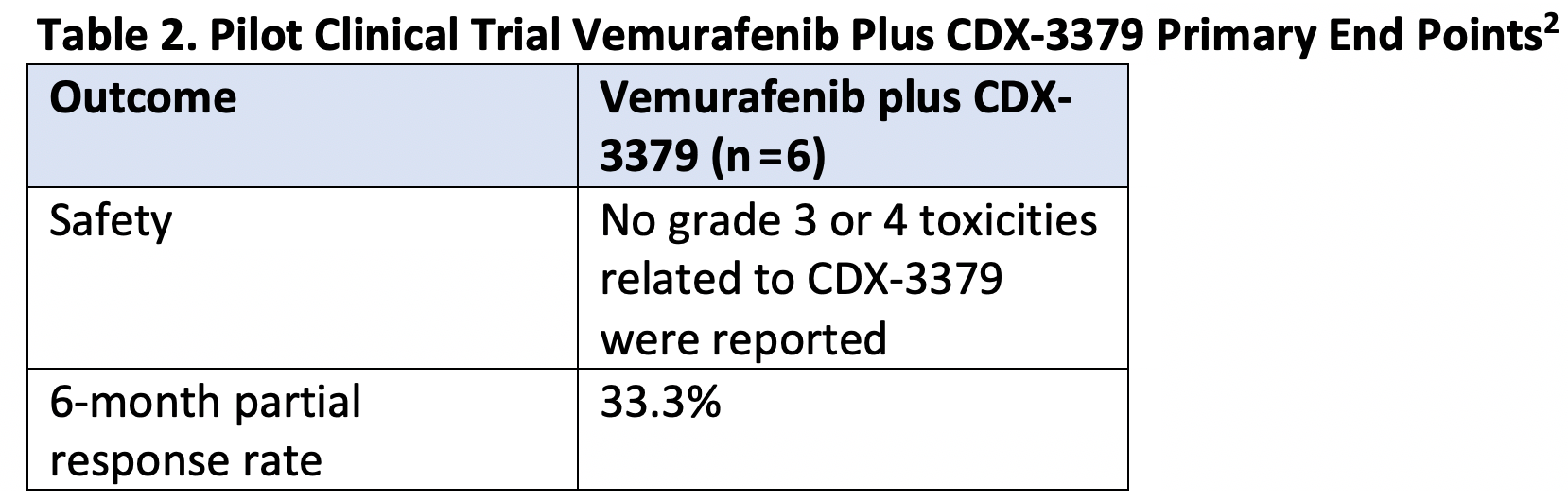
You recently published a pilot clinical trial looking for patients with BRAF-mutant, radioiodine-refractory thyroid cancers (NCT02456701). What was the rationale for this trial and what key data emerged regarding the combination’s efficacy? (Table 2)2
My colleagues and I demonstrated that in BRAF-mutant thyroid cancers, [first-generation] BRAF inhibitors of can be quite effective for a subset of patients where you can take tumors that are no longer avid for radioiodine and make them avid to that radioiodine making it an effective therapeutic again. But like all approaches, it was perfect in that it’s only a subset of patients.
The idea behind evaluating different combinations is to see if we could we increase the efficacy and broaden [the] use [of the available agents] to more patients with BRAF-mutant thyroid cancer. CDX-3379 targets HER3 and we have preclinical data demonstrating that when you treat BRAF-mutant thyroid cancers with BRAF inhibitors, you can get an upregulation of HER3 signaling. If you could abrogate that you can get more potent inhibition of the very pathways that we want to inhibit to restore iodine avidity.
This trial was a very small pilot trial, 6 patients with BRAF-mutant [disease], and we were able to demonstrate that the combination [of vemurafenib and CDX-3379] was safe and that we had good efficacy in terms of restoring redifferentiation. What’s needed in that field are larger studies and randomized trials to demonstrate the efficacy of redifferentiation for refractory patients, as well as randomized comparisons to know these combinations are better than the single agents.
Table 2. Pilot Clinical trial Vemurafenib Plus CDX-3379 Primary End Points
What is the importance of multidisciplinary management in this disease? Who else may be involved in patient treatment other than medical oncologists?
Multidisciplinary management is critical for patients with advanced thyroid cancers. First and foremost, it’s the surgeons who are, of course, important for upfront locally advanced disease control. Even for patients with tumors in dangerous locations sometimes surgeries can be considered.
The endocrinologist also helps manage TSH [thyroid stimulating hormone] suppression [and] nuclear medicine physicians [handle] radioactive iodine treatments. Radiation oncologists oftentimes [consider] palliative radiation or more definitive radiation for these patients. Then the medical oncologists are [handling] systemic therapy options, which have traditionally been relegated to [either] radioiodine refractory disease, anaplastic disease, mastoid disease, or recurrent metastatic disease.
Now there are new paradigms where clinical trials are evaluated with neoadjuvant use of drugs to see if we can improve surgical outcomes for patients. With the development of better drugs [and] better approaches it has increasingly become more [necessary for] multidisciplinary management of patients.
What challenges still lie ahead for developing thyroid cancer treatments?
Radioiodine refractory, recurrent, metastatic differentiated thyroid cancer; anaplastic thyroid cancer; these remain incurable diseases with limited options approved by the FDA. There is a need and a push to develop better, safer, and more effective treatments for those patients and there’s a continual push to better understand the biology, develop therapies, and conduct clinical trials.
Increasingly, [thyroid cancers have] gone from being considered rare diseases to diseases that we can well evaluate in clinical trials. In the settings where randomized trials may be necessary, now we can do those randomized trials in settings where we need to do larger phase 2 trials to reexplore signals for drugs. We need to pursue more definitive trials to understand the efficacy of the drugs that we’re developing.
References
- Brose MS, Robinson BG, Sherman SI, et al. Cabozantinib for previously treated radioiodine-refractory differentiated thyroid cancer: Updated results from the phase 3 COSMIC-311 trial. Cancer. Published online October 19, 2022. doi:10.1002/cncr.34493
- Tchekmedyian V, Dunn L, Sherman E, et al. Enhancing radioiodine incorporation in BRAF-Mutant, radioiodine-refractory thyroid cancers with vemurafenib and the anti-ErbB3 monoclonal antibody CDX-3379: results of a pilot clinical trial. Thyroid. 2022;32(3):273-282. doi:10.1089/thy.2021.0565








