Publication
Article
Data From ESMO 2023 Further Define Treatment Paradigm in Advanced Melanoma
Author(s):
Oncologists reviewed data from clinical trials in the field of melanoma that could add clarity to treatment standards and lead to improved patient care.
Meredith McKean, MD

During a recent OncLive Peer Exchange, expert oncologists reviewed long-term and preliminary data from key clinical trials in the field of melanoma that could add clarity to treatment standards and lead to improved patient care. During their discussion, the panelists paid particular attention to therapies for patients with BRAF-mutated disease and brain metastases. Findings were primarily presented during the European Society for Medical Oncology (ESMO) Congress 2023, which took place from October 20 to 24, 2023, in Madrid, Spain.
BRAF-Mutated Metastatic Melanoma
The panel began the discussion by emphasizing the importance of consistent genetic testing for patients with advanced melanoma, including immunohistochemistry and standardized next-generation sequencing, to identify patients who could be good candidates for a given therapy. Then they dove into updates from clinical trials in the field presented during ESMO 2023, first examining therapies for patients with BRAF-mutated melanoma.
"[BRAF] testing is helpful because psychologically when the patient first gets diagnosed, it's devastating and they're wondering what options they have," Helen Moon, MD, said. "Even if you don't use that piece of data right away, it colors your interaction with them and what you can offer that patient in terms of [treatment] options."
In the phase 2/3 RELATIVITY-047 trial (NCT03470922), investigators evaluated the combination of the anti-LAG-3 antibody relatlimab and the anti-PD-1 antibody nivolumab (Opdualag) compared with nivolumab (Opdivo) monotherapy in patients with previously untreated, unresectable, or metastatic melanoma. Previously published findings from RELATIVITY-047 showed that, at a median follow-up of 25.3 months, nivolumab plus relatlimab continued to display a benefit over nivolumab monotherapy; the median progression- free survival (PFS) was 10.2 months vs 4.6 months, respectively (HR, 0.81; 95% CI, 0.67- 0.97), and the median overall survival (OS) was not yet reached (NR) vs 33.2 months, respectively (HR, 0.82; 95% CI, 0.67-1.02).
In March 2022, the FDA approved the combination of nivolumab and relatlimab for the treatment of adult and pediatric patients 12 years or older with unresectable or metastatic melanoma. The regulatory decision was supported by findings from RELATIVITY-047.2
To further define the clinical characteristics of relatlimab plus nivolumab, investigators performed subgroup analyses, from which findings with 2 years of follow-up were presented during ESMO 2023. Data showed that the combination offered a PFS benefit over nivolumab monotherapy in subgroups of patients with baseline liver lesions (HR, 0.81; 95% CI, 0.53- 1.23) as well as those with baseline lung lesions (HR, 0.77; 95% CI, 0.57-1.05). Additionally, the combination also provided an OS benefit over nivolumab monotherapy in these groups, with respective HRs of 0.62 (95% CI, 0.37-1.03) and 0.72 (95% CI, 0.50-1.04). The central nervous system (CNS) metastasis-free survival in patients without CNS lesions at baseline also favored the combination arm (HR, 0.66; 95% CI, 0.34-1.27).1
Study authors concluded that relatlimab plus nivolumab provided an efficacy benefit over nivolumab monotherapy in patients with advanced melanoma regardless of number of baseline disease sites or the presence of baseline liver or lung lesions. They also noted that the combination displayed early signals of CNS activity and a manageable safety profile with a favorable risk to benefit ratio.1
“When I meet a frontline patient and I’m planning to do immune therapy, I first think, ‘Can I give you full-dose ipilimumab [Yervoy] plus nivolumab?’ If not, then certainly nivolumab plus relatlimab is my mainstay, as opposed to single-agent PD-1 [therapy],” Meredith McKean, MD, said. “Since the approval of relatlimab, I have given select patients singleagent– PD-1 [therapy]. What we saw in the RELATIVITY-047 study was that the toxicity [with nivolumab plus relatlimab] was very similar to anti–PD-1 monotherapy, but double the PFS, so I do like that option for some patients. [However], based on the long-term data [from the phase 3 CheckMate 067 trial] that we have with ipilimumab plus nivolumab, that’s still my go-to.”
“The patient has to decide, and we owe it to them to portray the toxicity as fully as we can,” John M. Kirkwood, MD, added. “[The key is] the detailed presentation of the toxicities of the two regimens [and choosing between] the maturity of the data for ipilimumab plus nivolumab vs the hope, but still early status for the relatlimab plus nivolumab trial data, and then it’s [the patient’s] decision. [However], ipilimumab plus nivolumab is the default; at this point in time I believe that is the gold standard.”
Ipilimumab with or without nivolumab was evaluated in patients with PD-1/L1 blockade refractory metastatic melanoma in the phase 2 S1616 study (NCT03033576). The study enrolled patients with metastatic melanoma who had received frontline anti-PD-1/L1 inhibition and experienced disease progression (n = 92). Patients were randomly assigned 3:1 to receive nivolumab plus ipilimumab or ipilimumab monotherapy. The primary end point was PFS; secondary end points included objective response rate (ORR), OS, and safety.3
Updated findings from the study showed that treatment with the combination resulted in a significant improvement in PFS compared with ipilimumab (HR, 0.63; 90% CI, 0.41-0.97, 1-sided P = .04). Additionally, the ORR was 28% (90% CI, 19%-38%) vs 9% (90% CI, 2%-25%), in the nivolumab plus ipilimumab vs the ipilimumab arm, respectively (1-sided P = .05). Investigators concluded that primary resistance to PD-1 blockade could be reversed in some patients with the combination of CTLA-4 and PD-1 blockade, although the change in intratumoral CD8 T-cell density had not reached significance.3
Another study that evaluated a combination approach in patients with locally advanced, unresectable, or metastatic melanoma was the phase 3 COLUMBUS trial (NCT01909453). The study enrolled patients with BRAF V6000E/ K-mutated disease who were treatment naive or had progressed following frontline immunotherapy, had an ECOG performance status of 1 or less, and had received no prior therapy with a BRAF and/or MEK inhibitor. COLUMBUS evaluated the combination of the BRAF inhibitor encorafenib (Braftovi) plus the MEK inhibitor binimetinib (Mektovi) vs encorafenib or vemurafenib (Zelboraf). The primary end point was PFS; secondary end points included ORR, OS, and safety.4
Findings from the 7-year update of COLUMBUS presented during ESMO 2023 demonstrated that patients in the combination (n = 192), vemurafenib (n = 191), and encorafenib (n = 194) arms experienced 7-year PFS rates of 21.2% (95% CI, 14.7%-28.4%), 6.4% (95% CI, 2.1%-14%), and 15.8% (95% CI, 9.3%- 23.8%), respectively, with the combination offering a PFS benefit over both vemurafenib (HR, 0.51; 95% CI, 0.39-0.66) and encorafenib (HR, 0.77; 95% CI, 0.60-0.99). Additionally, encorafenib plus binimetinib conferred a significant OS benefit vs vemurafenib (HR, 0.67; 95% CI, 0.53-0.84) and a slight benefit over encorafenib (HR, 0.93; 95% CI, 0.73-1.18; Table).4
Table. Efficacy Outcomes in 7-Year Update of the Phase 3 COLUMBUS Trial4
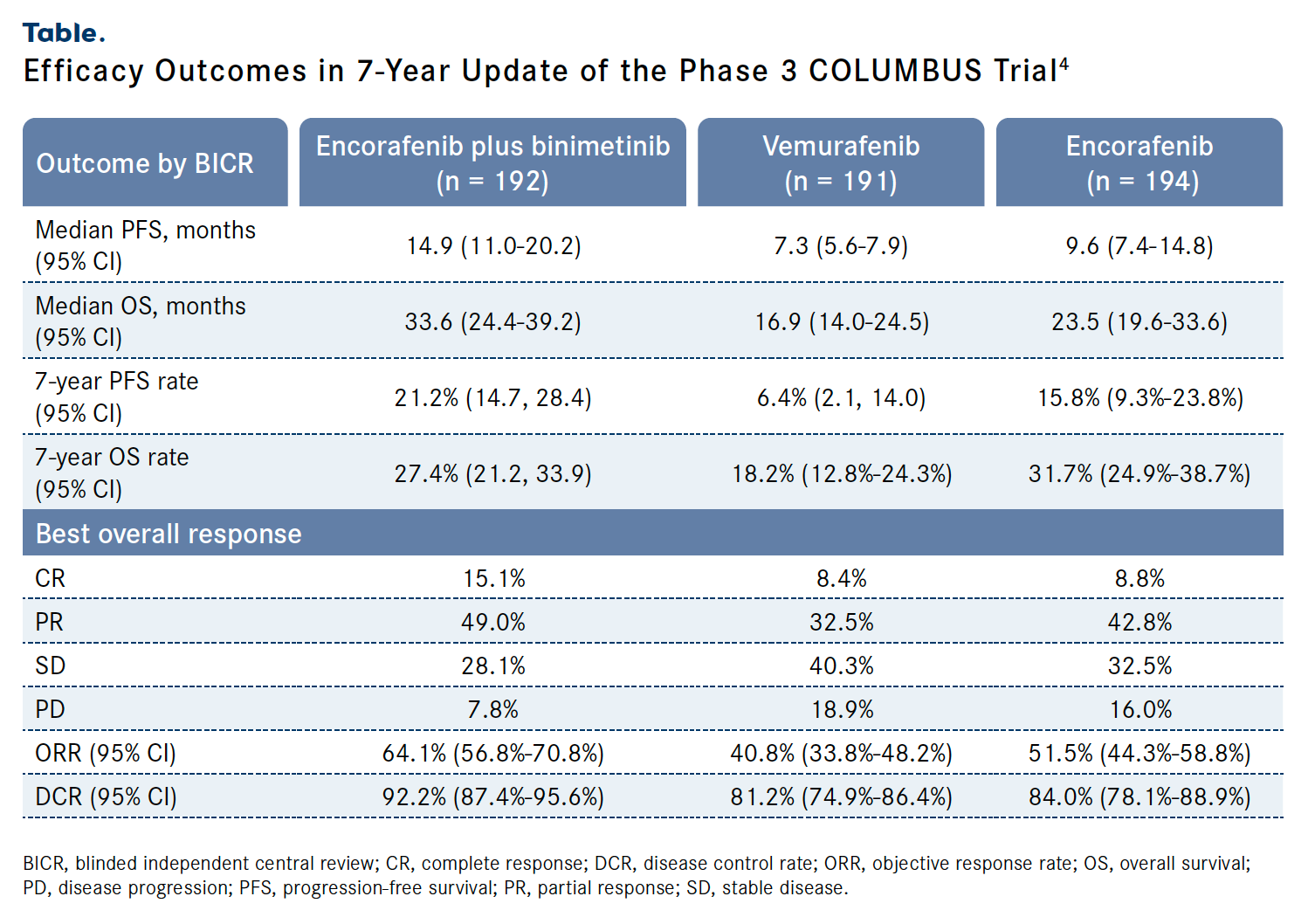
Investigators concluded that encorafenib plus binimetinib displayed long-term, sustained efficacy with more than 20% of patients free of progression. The combination also displayed a safety profile consistent with that previously reported of the agents with no new safety signals.4
Moreover, study authors also shared findings from the phase 2 REDUCTOR trial (EudraCT2013-002616-28) during ESMO 2023. REDUCTOR examined the clinical utility of neoadjuvant cytoreductive BRAF/MEK inhibition with dabrafenib (Tafinlar) plus trametinib (Mekinist) to allow for complete surgical resection in patients with previously unresectable, locally advanced BRAF-mutated melanoma. Previous findings from the study showed that major pathologic responses were observed in 50% of patients who received the combination and underwent surgery (n = 18).5
Results from the long-term survival follow-up of REDUCTOR presented during the conference showed that, at a median follow-up of 80.9 months (IQR, 38.6-89.7), the median relapse-free survival was 15.4 months (95% CI, 8.89-not reached [NR]) in patients who received the combination and underwent surgery (n = 18). Additionally, the median PFS was 12.4 months (95% CI, 8.68-NR); the median OS was NR, but the 1-, 3-, and 5-year OS rates were 100%, 85% (95% CI, 70.7%-100%), and 75% (95% CI, 58.2%-96.6%), respectively.5
“In those patients who relapse with resectable, regional disease we have a second gun loaded in our holster [following recurrence] because we have PD-1–[directed] interventions that are highly effective,” Kirkland said. “The modality of circulating tumor DNA tracking is something that we will use more and more in those scenarios. Also, in [terms of] monitoring the patient with BRAF/MEK treatment, we will probably learn more about this in the next few years, which will allow us to intelligently approach this going forward.”
“That’s going to be a big question: response in the metastatic setting with prior exposure to these agents in the adjuvant setting. We don’t have formal data on this exactly, but I would hope that the longer gap in between the adjuvant and the metastatic treatment can keep the patient sensitized to another course of what they’ve had,” Michael Postow, MD, noted.
Advanced Melanoma With Brain Metastases
The panel transitioned the conversation to discuss treatment options for patients with melanoma brain metastases. Melanoma is among the solid tumors most likely to lead to brain metastases; greater than 25% of patients have metastatic disease at the point of diagnosis and more than 15% have brain metastases. Notably, 75% of patients with melanoma have been found to die with brain metastases, according to autopsy reports. Brain metastases in melanoma are also associated with worse outcomes and, despite treatment advances, there is no optimal treatment available to manage melanoma-related brain metastases.6
During ESMO 2023, investigators presented findings from the 7-year follow-up analysis of the phase 3 NIBIT-M2 trial (NCT02460068), which enrolled patients with metastatic melanoma with active and untreated asymptomatic brain metastases; patients were not allowed to have received prior systemic therapy for advanced disease. The study randomly assigned patients 1:1:1 to receive fotemustine (arm A; n = 23), ipilimumab plus fotemustine (arm B; n = 26), or ipilimumab plus nivolumab (arm C; n = 27). The primary end point was OS; secondary end points included PFS, intracranial and global immune-related (ir) ORR, and safety.7
At the May 1, 2023, data cutoff, at a median follow-up of 67 months (IQR, 42-79), the median OS in arms A, B, and C was 8.5 months (95% CI, 4.8-12.2) vs 9.1 months (95% CI, 4.3-20.4) vs 29.2 months (95% CI, 12.9-65.1), respectively. The 5-year OS rates were 10%, 10%, and 43%, respectively; notably, 43% of patients remained alive in arm C at 7 years. The median PFS was 3.0 months (95% CI, 2.3-3.6) vs 3.5 months (95% CI, 1.2-5.9) vs 8.7 months (95% CI, 5.7-17.6), respectively.7
Notably, patients who received ipilimumab plus nivolumab experienced a significant benefit in terms of intracranial irORR; the rates were 0% vs 19.2% vs 44.4% in arms A, B, and C, respectively. Additionally, the ir disease control rates (DCRs) were 21.7% vs 34.6% vs 55.5%, respectively. In terms of global response, the irORRs were 0% vs 23.1% vs 44.4%, respectively. The global response irDCRs were 17.4% vs 30.8% vs 55.5%, respectively.
“If a patient has truly asymptomatic disease and has a BRAF mutation, you’re in this fortunate situation where you have a lot of options,” Sapna Patel, MD, said. “You have 3 generations of BRAF/MEK inhibitors, and you have fulldose ipilimumab at 3 mg with nivolumab at 1 mg. We’ve never compared the head-to-head activity of one group of drugs vs another, [but] we feel comfortable with the immunotherapy responses and the durability. [Data from] the NIBIT-M2 study showed that those asymptomatic patients are doing quite well in the long run. Immunotherapy [with ipilimumab plus nivolumab] remains our go-to for them, and we hope that that becomes a long-[term] treatment and that they get the benefit of that treatment- free interval.“
Finally, in the phase 2 SECOMBIT trial (NCT02631447) study authors compared a targeted approach with encorafenib plus binimetinib (arm A) with the immunotherapy combination of ipilimumab plus nivolumab (arm B), and evaluated a third arm in patients who received encorafenib plus binimetinib with a preplanned switch to ipilimumab plus nivolumab at 8 weeks then back to encorafenib plus binimetinib after progression. Patients in arm A switched to the arm B regimen following progression and vice versa. The primary end point was OS; secondary end points included PFS, ORR, and safety.8
At the May 31, 2023, data cutoff, at a median follow-up of 56 months (IQR, 48-63), findings presented during ESMO 2023 demonstrated that the 5-year OS rates in arms A (n = 69), B (n = 69), and C (n = 68) were 45% vs 52% vs 57%, respectively. An exploratory analysis showed that patients in both arms B (HR, 0.75; 95% CI, 0.46-1.22; P = .25) and arm C (HR, 0.71; 95% CI, 0.43-1.16; P = .17) experienced an OS benefit vs those in arm A.8
The 5-year PFS rates in arms A, B, and C were 27% vs 50% vs 50%, respectively. Data from an exploratory analysis showed that both arms B (HR, 0.61; 95% CI, 0.39-0.94; P = .03) and arm C (HR, 0.58; 95% CI, 0.37-0.90; P = .02) achieved a significant PFS benefit over arm A.8
The authors concluded that, although the study was limited by its phase 2 nature and small patient population, patients treated in arm A were at a higher risk of developing brain metastases than those in arms B and C. The study is ongoing to evaluate long-term outcomes and additional biomarkers, they noted.8
References
- Long GV, Hodi FS, Lipson EJ, et al. Nivolumab (NIVO) plus relatlimab (RELA) vs NIVO in previously untreated metastatic or unresectable melanoma: 2-year subgroup analyses from RELATIVITY-047. Ann Oncol. 2023;34(suppl 2):S664-S665. doi:10.1016/j.annonc.2023.09.2237
- FDA approves Opdualag for unresectable or metastatic melanoma. News release. FDA. Updated March 21, 2022. Accessed December 19, 2023. https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-opdualag-unresectable-or-metastatic-melanoma
- VanderWalde A, Bellasea SL, Kendra KL, et al. Ipilimumab with or without nivolumab in PD-1 or PD-L1 blockade refractory metastatic melanoma: a randomized phase 2 trial. Nature Med. 2023;29(9):2278-2285. doi:10.1038/s41591-023- 02498-y
- Schadendorf D, Dummer R, Flaherty KT, et al. COLUMBUS 7-year update: a randomized, open-label, phase III trial of encorafenib (enco) + binimetinib (bini) vs vemurafenib (vemu) or enco in patients (pts) with BRAF V600–mutant melanoma. Ann Oncol. 2023;34(suppl 2):S670-S671. doi:10.1016/j.annonc.2023.09.2247
- Burgers FH, Blankenstein S, Rohaan M, et al. Long-term survival follow-up from the REDUCTOR trial: neoadjuvant cytoreductive treatment with BRAF/MEK inhibition of prior unresectable regionally advanced melanoma to allow complete surgical resection. Ann Oncol. 2023;34(suppl 2):S662. doi:10.1016/j. annonc.2023.09.2233
- Wilson TG, Winter H, Taylor H, Herbert C. Treating brain metastases in melanoma: what is the optimal CNS-directed and systemic management? J Radiosurg SBRT. 2021;7(4): 279-285.
- Di Giacomo AM, Sileni VC, Del Vecchio M, et al. Nivolumab plus ipilimumab in melanoma patients with asymptomatic brain metastases: 7-year outcomes and quality of life from the multicenter phase III NIBIT-M2 trial. Ann Oncol. 2023;34(suppl 2):S653-S654. doi:10.1016/j.annonc.2023.09.2218
- Ascierto PA, Mandala M, Fercucci PF, et al. Brain metastases and survival evaluation in the SECOMBIT trial. Ann Oncol. 2023;34(suppl 2):S651-S700. doi:10.1016/annonc/ annonc1329









