Publication
Article
Immunotherapy-Based Approaches Make Waves in Bladder Cancer
Author(s):
Experts highlight clinical trial updates in muscle-invasive bladder cancer, frontline treatment options in metastatic urothelial carcinoma, and more.
Elizabeth Plimack, MD, MS, FASCO

Bladder cancer is the sixth most frequently occurring cancer in the United States, with a global mortality rate of 2.9%. The treatment paradigm in most subtypes of the disease was defined by chemotherapy for many years; however, recent updates from clinical studies have spurred hope that more modern agents, such as immunotherapeutics and antibody-drug conjugates (ADCs), can be added to the treatment armamentarium and spare patients from the toxicity that is often associated with traditional cytotoxic agents while maintaining, or even improving, efficacy.1
During a recent OncLive® Peer Exchange, a panel of expert clinicians in the field of genitourinary oncology discussed updated findings from key clinical trials presented during the 2024 American Society of Clinical Oncology Genitourinary Cancers Symposium (ASCO GU 2024), which was held in January in San Francisco, California. They highlighted key clinical trial updates in muscle-invasive bladder cancer, frontline treatment options in metastatic urothelial carcinoma, and the development of new approaches for patients in the second line and beyond.
Neoadjuvant and Adjuvant Therapy in Muscle-Invasive Bladder Cancer
The panelists began their conversation by discussing updates in muscle-invasive bladder cancer in the neoadjuvant setting, a setting in which chemotherapy, despite its limited activity, is the established treatment modality.2 “[In the neoadjuvant space] the data are certainly exciting,” Matthew Milowsky, MD, FASCO, said. “There’s a lot of integration of immune checkpoint blockade into the neoadjuvant setting, as well as other novel agents. A number of studies have looked at immune checkpoint blockade alone or in combination with chemotherapy.”
One such study of neoadjuvant immunotherapy that was presented during ASCO GU 2024 was the phase 2 GDFATHER-NEO trial (NCT06059547). GDFATHER-NEO is a multicenter, single-blinded study evaluating the GDF-15 neutralizing IgG4 monoclonal antibody visugromab (CTL-002) in combination with nivolumab (Opdivo) in patients with muscle-invasive bladder cancer who are ineligible for or refuse neoadjuvant cisplatin-based chemotherapy. The primary end points were pathologic complete response (pCR) and radiologic response rate; secondary end points include safety, event-free survival (EFS), and overall survival (OS).2
Preclinical findings showed that GDF-15 neutralization showed promise in overcoming tumor immune escapes and immunosuppression mechanisms. Additionally, in the phase 1 study, the combination proved to be safe and effective with deep and durable responses in PD-1/PD-L1 relapsed/refractory metastatic solid tumors. The present study is recruiting patients and phase 2 data have not been published.
Another immunotherapy-based approach is being evaluated in patients with muscle-invasive bladder cancer in the phase 2 NURE-Combo trial (NCT04876313), which is examining nivolumab in combination with nab-paclitaxel (Abraxane) followed by radical cystectomy and adjuvant nivolumab. The primary end point is pCR; secondary end points include EFS, safety, and major pathological response.3
Patients who received the study treatment (n = 31) achieved a ypT0N0 response rate of 38.7% (95% CI, 22%-56%) and 73.3% (95% CI, 55%-87%) achieved a yp≤1N0 response. Notably, no disease progression occurred during neoadjuvant treatment; at a median follow-up of 12 months (IQR, 9-17.5) the 12-month EFS rate was 89.1% (95% CI, 78.3%-100%). Study authors concluded that nivolumab plus nab-paclitaxel represents a safe and effective perioperative strategy in muscle-invasive bladder cancer and that their findings could expand opportunities for chemotherapy combinations in cisplatin-ineligible patients.
“Right now, we’re still leaning on cytotoxic chemotherapy per NCCN [National Comprehensive Cancer Network] guidelines,” Elizabeth Plimack, MD, MS, FASCO, commented. “There’s a preference [for] dosedense accelerated MVAC [methotrexate, vinblastine, doxorubicin, and cisplatin] based on [data from the phase 3] VESPER trial [NCT01812369] and pT0 rates. [Additionally], the need for long-term time-to-event end points is critical in these studies. We’re trying to cure people and we need to prove that we’re doing that. There’s [also] a lot of enthusiasm for novel agents, including immunotherapy in this space.”
In the adjuvant setting of muscle-invasive bladder cancer, updated findings from 2 phase 3 studies were reported during ASCO GU 2024. In the phase 3 AMBASSADOR trial (NCT03244384), investigators compared adjuvant pembrolizumab (Keytruda) with observation in patients with muscle-invasive urothelial carcinoma who underwent radical surgery 4 to 16 weeks prior to receiving study treatment. The coprimary end points were OS and disease-free survival (DFS).4
The median DFS in the pembrolizumab arm (n = 354) was 29.0 months (95% CI, 21.8-not reached [NR]) compared with 14.0 months (95% CI, 9.7-20.2) in the observation arm (n = 348; HR, 0.69; 95% CI, 0.54-0.87; P = .001). However, the OS end point was not met at the interim analysis; the median OS was 50.9 months (95% CI, 43.8-NR) vs 55.8 months (95% CI, 53.3-NR), respectively (HR, 0.98; 95% CI, 0.76-1.26; P = .884). The study authors concluded that their findings support adjuvant pembrolizumab as a new therapeutic option in this patient population and additional follow-up is ongoing for the final DFS and OS analyses.
Investigators also presented findings from DFS analyses of the phase 3 CheckMate 274 trial (NCT02632409), which compared adjuvant nivolumab with surveillance in patients with muscle-invasive bladder cancer at high risk after radical cystectomy. Only patients who underwent surgery within the past 120-day window were eligible for enrollment. The primary end point was DFS in the intent-to-treat (ITT) population and DFS in patients with a PD-L1 level of at least 1%.5 The median DFS in the ITT population was 22.0 months (95% CI, 18.8-36.9) with nivolumab (n = 353) compared with 10.9 months (95% CI, 8.3-15.2) in the surveillance arm (n = 356; HR, 0.71; 95% CI, 0.58-0.86). Additionally, adjuvant nivolumab provided a significant benefit over surveillance among patients in the PD-L1–positive subgroup (HR, 0.52; 95% CI, 0.37-0.72). Due to a lack of mature OS data, investigators utilized mixture cure models to estimate the impact of nivolumab on long-term survivorship; data from the models demonstrated that adjuvant nivolumab led to an estimated 6% to 9% absolute increase in the cure rate in the ITT population and a 23% to 25% absolute increase in the cure rate in the PD-L1–positive population compared with radical cystectomy alone.
Frontline and Maintenance Therapy for Metastatic Urothelial Carcinoma
The panelists transitioned their discussion to updates in metastatic urothelial carcinoma presented during ASCO GU 2024, starting with the phase 3 EV-302/KEYNOTE KN-A39 trial (NCT04223856), which compared enfortumab vedotin-ejfv (Padcev) plus pembrolizumab with the long-time standard-of-care platinum-based chemotherapy in patients with untreated locally advanced metastatic urothelial carcinoma. Primary findings from the study, which were presented during the European Society for Medical Oncology (ESMO) Congress in October 2023, demonstrated that the combination significantly improved progression-free survival (PFS) over chemotherapy (HR, 0.45; 95% CI, 0.38-0.54; P < .00001) and OS (HR, 0.47; 95% CI, 0.38-0.58; P < .00001).6
“EV-302 stole the limelight; it was an international randomized trial of 900 patients with locally advanced or metastatic urothelial carcinoma and this time they could have been eligible or not eligible for cisplatin,” Neeraj Agarwal, MD, FASCO, said. “The coprimary end points were PFS and OS. The most striking result was the doubling of OS at 31.5 months vs 16.1 months, with a 53% reduction in the risk of progression or death with enfortumab vedotin [plus] pembrolizumab. I don’t think we have seen these kinds of exciting results ever in our field. So this is obviously fantastic news for our patients.”
Findings from subgroup analyses presented during ASCO GU 2024 showed that the OS benefit displayed by enfortumab vedotin plus pembrolizumab over standard-of-care chemotherapy was consistently observed across prespecified subgroups. The most pronounced benefit was observed among patients with an ECOG performance status of 0 (HR, 0.36; 95% CI, 0.25-0.53) and those from Europe (HR, 0.40; 95% CI, 0.28-0.56). Study authors concluded that the results of the analyses supported the findings of the primary analysis, indicating the combination of enfortumab vedotin plus pembrolizumab is a new standard of care in frontline locally advanced or metastatic urothelial carcinoma (Table).7
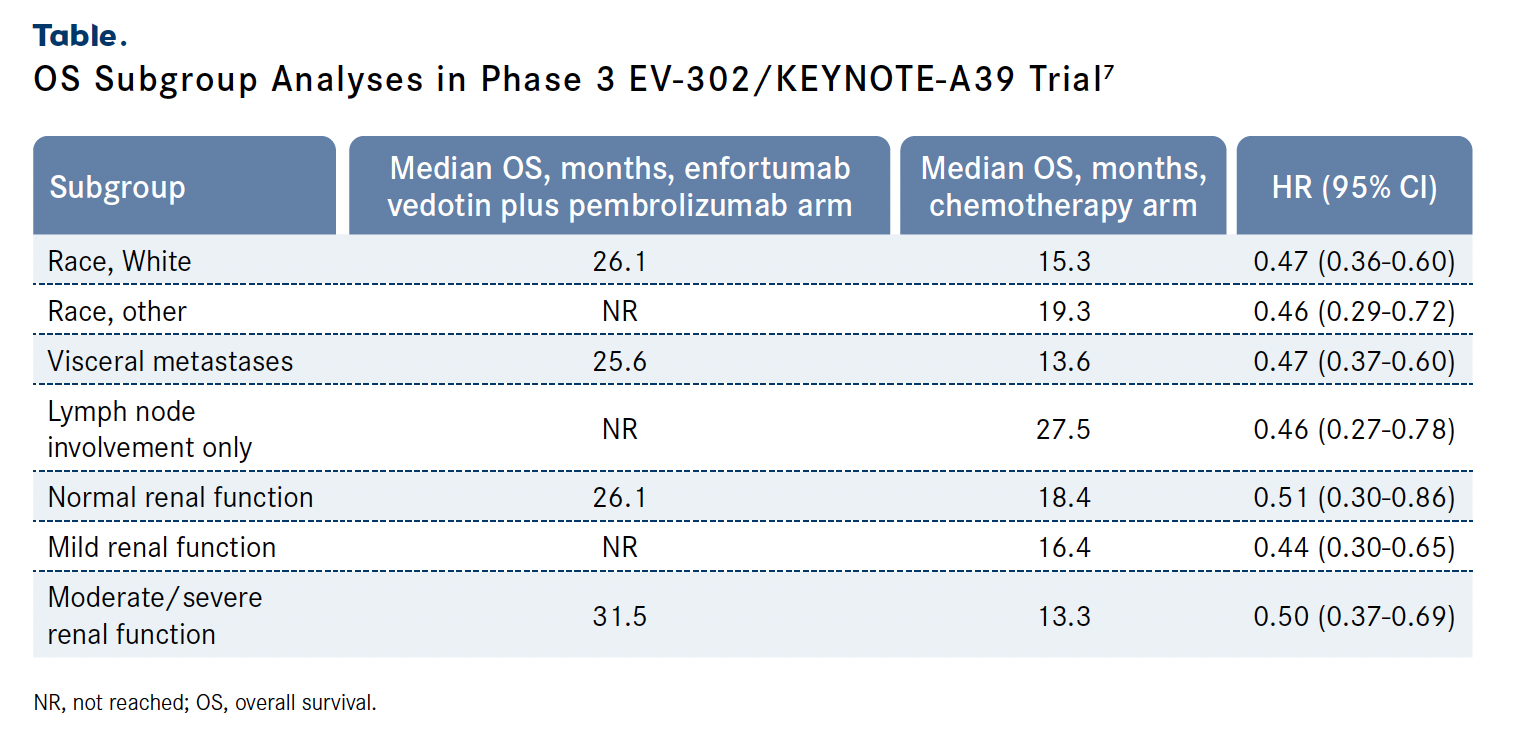
Shortly after the presentation at ESMO 2023, in December 2023, the FDA approved the combination of enfortumab vedotin and pembrolizumab for the treatment of patients with locally advanced or metastatic urothelial cancer.8
The panelists also highlighted updated findings from the phase 3 JAVELIN Bladder 100 trial (NCT02603432), which compared maintenance treatment with the PD-L1–directed monoclonal antibody avelumab (Bavencio) plus best supportive care with best supportive care alone in patients with advanced urothelial carcinoma who did not experience disease progression after completing frontline treatment with platinum-containing chemotherapy. Previously reported topline findings from the study showed that, after a minimum follow-up of 2 years, the addition of avelumab significantly prolonged OS vs best supportive care alone, at 23.8 months vs 15.0 months, respectively (HR, 0.76; 95% CI, 0.63-0.91; P = .0036).9
In a patient-reported outcomes analysis presented at ASCO GU 2024, investigators noted that the addition of avelumab to best supportive care did not negatively affect patient quality of life. Patient-reported outcome scores remained stable throughout treatment, with a least-squares mean change from baseline of –0.05 (95% CI, –0.63 to 0.52), 0.68 (95% CI, 0.46-0.91), and –0.02 (95% CI, –0.04 to 0.0) in physical disease–related symptoms, emotional disease-related symptoms, and EQ-5D-5L index scores, respectively, among those who received at least 1 year of avelumab.
Also during ASCO GU 2024, investigators presented findings from the observational, retrospective PATRIOT-II study. PATRIOT-II collected data from 160 patients with metastatic urothelial carcinoma across 37 sites in the United States who received first-line avelumab maintenance therapy in routine clinical practice. Findings from the study showed that the real-world results were in line with those of JAVELIN Bladder 100; the median real-world OS at a minimum of 24 weeks post frontline avelumab initiation was 24.4 months (SE, 2.0; 95% CI, 20.4-28.4) and 30.5 months (SE, 3.6; 95% CI, 23.4-37.6) from the start of platinum-based chemotherapy.10
“We used to talk about [patients being] cisplatin fit [vs] cisplatin unfit. [Now] I think more about [them being] combination therapy eligible vs combination ineligible, Evan Y. Yu, MD, said. “I look at ECOG performance status, and if it’s 0 to 2, they have a GFR [glomerular filtration rate] greater than 30, and adequate organ function, in general they are a combination patient. Under those circumstances, most patients can receive enfortumab vedotin in combination with pembrolizumab based on EV-302 [data]. If they are not eligible for enfortumab vedotin, usually that is a patient with significant neuropathy or a patient with very poorly controlled diabetes. Neuropathy would represent an issue as well with the [phase 3 CheckMate]901 [NCT03036098] regimen based on the use of cisplatin, so I would lean more on the JAVELIN Bladder 100 regimen [after treatment with] gemcitabine, carboplatin, and maintenance nivolumab.”
Later-Line Therapies for Metastatic Urothelial Carcinoma
The panelists concluded their conversation with a brief discussion of ongoing and upcoming early-phase studies in patients with pretreated metastatic urothelial carcinoma. “Most of us were using platinum in the first line for metastatic disease, then probably using immune-oncology agents like pembrolizumab in the second line, and enfortumab vedotin in the third line. Now that you take your second- and third-line [agents], enfortumab vedotin and pembrolizumab, combine them up front, [and] move them to the first line like we’ve been talking about, that leaves a real question mark as to what we should be doing [in the] second line,” Yu commented.
The experts highlighted an ongoing phase 1/2 trial (NCT04863885) of ipilimumab (Yervoy) plus nivolumab and sacituzumab govitecan-hziy (Trodelvy) in patients with metastatic urothelial carcinoma who are ineligible for cisplatin therapy. The study is enrolling treatment-naive patients with an ECOG performance status of 1 or less who did not receive prior chemotherapy. The primary end points are safety and determining the maximum tolerated and recommended phase 2 doses; secondary end points include objective response rate (ORR), PFS, OS, and duration of response.11
Finally, the panelists discussed preliminary findings from the phase 1 TROPION-PanTumor01 study (NCT03401385), which is examining the ADC datopotamab deruxtecan in patients with unresectable advanced or metastatic urothelial cancer following at least 1 prior line of therapy. The primary end point was safety; secondary end points included ORR and disease control rate (DCR).12
At a median follow-up of 9.1 months (range, 5-17), preliminary findings from the study showed that response-evaluable patients (n = 26) achieved a confirmed ORR of 19.2% (95% CI, 6.6%- 39.4%), including a 3.8% complete response rate. The DCR was 80.8% (95% CI, 60.6%-93.4%). In terms of safety, datopotamab deruxtecan was tolerable, with a grade 3 treatment-emergent adverse effect rate of 33%; the agent is being further evaluated in patients with urothelial cancer in the phase 1/2 TROPION-PanTumor02 (NCT05460273) and the phase 2 TROPIONPanTumor03 (NCT05489211) clinical trials.
“We’re not curing patients, and we do have an opportunity to cure patients potentially as we move these therapies earlier, whether it be targeting HER2 earlier, using enfortumab vedotin plus pembrolizumab earlier, or different combinations of immunotherapy and ADCs in the adjuvant setting or targeted therapeutics,” Milowsky said in conclusion. “This is really the way and has been borne out in other diseases as well: RNA vaccine-based approaches in the adjuvant setting in combination with immune checkpoint blockade. These things are happening, but the faster we can make them happen…is going to ultimately translate to a higher cure rate for our patients overall.”
References
- Shafique MA, Haseeb A, Siddiq MA, Mussarat A, Rangwala HS, Mustafa MS. Current and emerging treatments for urothelial carcinoma: a focus on enfortumab vedotin. Cancer Manag Res. 2023;15:699-706. doi:10.2147/CMAR.S418009
- Necchi A, Iacovelli R, Di Maio M, et al. Neutralizing GDF-15 in muscle-invasive bladder cancer (MIBC): a neoadjuvant immunotherapy trial of visugromab (CTL-002) in combination with the anti-PD1 antibody nivolumab (GDFather-Neo). J Clin Oncol. 2024;42(suppl 4):TPS712. doi:10.1200/JCO.2024.42.4_suppl. TPS712
- Mercinelli C, Basile G, Raggi D, et al. First results of NURECombo: a phase 2 study of neoadjuvant nivolumab (NIVO) and nab-paclitaxel (ABX) followed by postsurgical adjuvant NIVO in patients (pts) with muscle-invasive bladder cancer (MIBC). J Clin Oncol. 2024;42(suppl 4):610. doi:10.1200/ JCO.2024.42.4_suppl.610
- Apolo AB, Ballman KV, Sonpavde GP, et al. AMBASSADOR Alliance A031501: phase III randomized adjuvant study of pembrolizumab in muscle-invasive and locally advanced urothelial carcinoma (MIUC) vs observation. J Clin Oncol. 2024;42(suppl 4):LBA531. doi:10.1200/JCO.2024.42.4_suppl. LBA531
- Geynisman DM, Yates G, Chepynoga K, et al. Estimating the impact of adjuvant treatment with nivolumab on long-term survivorship rates compared with surveillance: analyses of disease-free survival (DFS) from the phase 3 CheckMate-274 trial. J Clin Oncol. 2024;42(suppl 4):528. doi:10.1200/ JCO.2024.42.4_suppl.528
- Powles TB, Valderrama BP, Gupta S, et al. EV-302/KEYNOTE- A39: open-label, randomized phase III study of enfortumab vedotin in combination with pembrolizumab (EV+P) vs chemotherapy (chemo) in previously untreated locally advanced metastatic urothelial carcinoma (la/mUC). Ann Oncol. 2023;34(suppl 2):S1340. doi:10.1016/j.annonc.2023.10.106
- Van Der Heijden MS, Powles TB, Gupta S, et al. Enfortumab vedotin (EV) in combination with pembrolizumab (P) versus chemotherapy in previously untreated locally advanced metastatic urothelial carcinoma (la/mUC): subgroup analyses results from EV-302, a phase 3 global study. J Clin Oncol. 2024;42(suppl 4):LBA530. doi:10.1200/JCO.2024.42.4_suppl. LBA530
- FDA approves enfortumab vedotin-ejfv with pembrolizumab for locally advanced or metastatic urothelial cancer. FDA. December 15, 2023. Accessed March 22, 2024. https://www.fda.gov/ drugs/resources-information-approved-drugs/fda-approvesenfortumab- vedotin-ejfv-pembrolizumab-locally-advancedor- metastatic-urothelial-cancer
- Grivas P, Aragon-Ching JB, Bellmunt J, et al. Avelumab first-line maintenance (1LM) for advanced urothelial carcinoma (aUC): long-term patient-reported outcomes (PROs) in the phase 3 JAVELIN Bladder 100 trial. J Clin Oncol. 2024;42(suppl 4):581. doi:10.1200/JCO.2024.42.4_suppl.581
- Grivas P, Barata PC, Moon HH, et al. Avelumab first-line maintenance therapy for locally advanced/metastatic urothelial carcinoma: results from the real-world US PATRIOT-II study. J Clin Oncol. 2024;42(suppl 4):697. doi:10.1200/ JCO.2024.42.4_suppl.697
- Jain RK, Yang Y, Chadha J, et al. Phase I/II study of ipilimumab plus nivolumab (IPI-NIVO) combined with sacituzumab govitecan in patients with metastatic cisplatin-ineligible urothelial carcinoma. J Clin Oncol. 2024;42(suppl 4):TPS713. doi:10.1200/ JCO.2024.42.4_suppl.TPS713
- Lisberg A, Drakaki A, Meric-Bernstam F, et al. Datopotamab deruxtecan in locally advanced/metastatic urothelial cancer: preliminary results from the phase 1 TROPION PanTumor01 study. J Clin Oncol. 2024;42(suppl 4):603. doi:10.1200/ JCO.2024.42.4_suppl.603








