
The challenges of assessing COVID-19 vaccines shows that events in the real world may differ from those of a formal objective scientific analysis, especially in a setting where such evaluations of necessity involve very small numbers.

Your AI-Trained Oncology Knowledge Connection!


Maurie Markman, MD, is president of Medicine & Science at City of Hope Atlanta, Chicago, and Phoenix

The challenges of assessing COVID-19 vaccines shows that events in the real world may differ from those of a formal objective scientific analysis, especially in a setting where such evaluations of necessity involve very small numbers.

Although the spectacular success associated with the development of several safe and highly efficacious vaccines and therapies for COVID-19 has once again confirmed the remarkable impact of advancements on public and individual health, we must also acknowledge recent stunning examples of the failure of scientifically oriented government agencies to provide objectively valid nonpolitical recommendations, policies, and conclusions.

The current endorsement of 3 COVID-19 vaccines in the United States, with several additional products pending FDA review, in less than 1 year from the identification of the structure of the COVID-19 virus is simply remarkable.

In the cancer arena, COVID-19 information problems highlight the critical role of clear, honest, and effective communication with the public, patients, and their families regarding the increasing complexity of a multitude of topics related to malignant disease and its management.

Evolving data provide provocative support for the relevance of an optimistic vs a pessimistic perspective in influencing outcomes in serious malignant conditions.

When one is dealing with the topic of cancer, its treatment and consequences, language that clinicians and members of the cancer research community use may have an even greater impact.

Formerly, small cell lung cancer was generally considered more chemotherapy sensitive in the short term but also associated with an overall inferior survival outcome; however, the difference in prognosis compared with metastatic non–small cell lung cancer was measured in months—rarely longer.

Maurie Markman, MD, discusses the need for comparative clinical trials with PARP inhibitors in ovarian cancer.

The importance of objectively valid data is well established in clinical medicine. Such data include an accurate recording of a patient’s clinical history; evaluation of signs and symptoms of illness; and measurement of various routine indicators, such as granulocyte and platelet counts, serum glucose, electrolytes, and liver function tests.

Although recent benefits do not pertain to all cancers and “cure” remains a relatively uncommon event, oncologists have an increasing number of molecularly targeted and immunotherapeutic strategies to employ based on objectively meaningful clinical trial outcomes.

Maurie Markman, MD, discusses remaining questions regarding human papillomavirus–negative cervical cancer.

In a pandemic, the public and its leaders yearn for relatively simple answers that can lead to solutions and forceful actions such as preventing serious infection and hospitalizations, treating active illness, and developing safe and effective vaccines quickly made available to the public.

Although PARP inhibitors are generally reasonably well tolerated, certainly compared with platinum and other routinely employed cytotoxic antineoplastic drugs, the majority of patients receiving PARP inhibitors in multiple reported clinical trials reported low-grade nausea and fatigue.

Maurie Markman, MD, discusses the utility of PARP inhibitors in ovarian cancer.
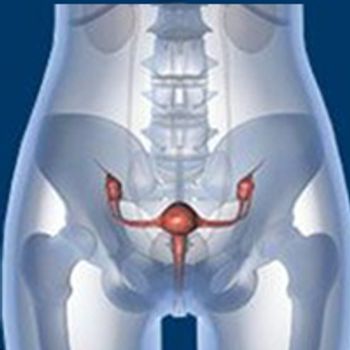
Despite solid evidence that human papillomavirus vaccination is remarkably effective in preventing persistent infection by HPV types that are known to be responsible for more than 70% to 80% of cervical cancer cases worldwide, this strategy’s utility in substantially reducing the subsequent development of the malignancy itself remained an open question.

The fundamental objectivity of major scientifically oriented public health agencies has been called into question, while the issue of what constitutes sufficient evidence for a therapeutic strategy to be considered “standard” is also being challenged.

In the realm of medicine, we see enormous opportunities for truly novel technologies to revolutionize the health and welfare of all members of society.

Because it is likely that COVID-19 will be around for a considerable period of time, it is hoped that all involved in the clinical research establishment can view this experience as a process that needs substantial improvement.

Several developments emphasize the increasing clinical relevance of germline testing within the oncology arena and the need for associated genetic counseling.

The coronavirus disease 2019 pandemic has raised the public profile of experts in infectious disease, epidemiology, and public health.

These are disquieting times for clear and decisive messaging regarding vaccines.

Precision medicine should be seen as being synergistic―or at a minimum additive―to population medicine.

The explosion of COVID-19 studies illustrates questions that have plagued oncology research.

At this moment, we should consider what it will take for the scientific community as a collective entity to self-regulate far more forcefully so that the actions of a limited number of its members do not squander the well-deserved awakening of public trust in the entire scientific enterprise.

From the earliest moments of medical school instruction, through residency and fellowship training, physicians are taught the primacy of the randomized phase 3 trial in the hierarchy of evidence-based medicine.

From the earliest moments of medical school instruction, through residency and fellowship training, physicians are taught the primacy of the randomized phase 3 trial in the hierarchy of evidence-based medicine.

What was simply unimaginable just a few weeks earlier regarding the potential impact of an infectious illness in the United States on jobs, schools, the economy, and personal and family safety has become a stream of daily pronouncements about the severity of the pandemic and how best to respond.

The conduct of randomized trials in surgical oncology, although highly appealing in concept, may be problematic, especially in complex settings where the skills, experience, and clinical judgment of individual surgeons and their institutions may vary greatly.

This has been a difficult time for public health policy and regulatory organizations struggling to deal with rapidly changing and unquestionably serious societal health-related issues and concerns. The list of problems these agencies must tackle is growing, and so are the questions about the strategies that should be used to address these threats.

Segments of the population have apparently rejected the well-documented clinical utility of vaccination for protecting individual and public health. An immediate specific concern is the contentious matter of measles vaccination, which has been well-reported in the lay press.