Publication
Article
A Growing Arsenal of NSCLC Treatments Take Aim at Oncogenic Fusions
Author(s):
A panel of experts discuss their perspectives on testing and targeted therapy for actionable mutations in non–small cell lung cancer.
Benjamin P. Levy, MD

Recent FDA approvals of several tumor-agnostic drugs are shifting the cancer treatment paradigm from traditional organ/ histology-directed therapies to a precision cancer medicine approach using biomarkers to guide treatment.1 One such biomarker is oncogenic fusions, the detection and targeting of which has been hailed as one of the most important breakthroughs in oncology over the past century.2
Patients with non–small cell lung cancer (NSCLC) are among the major beneficiaries of this approach. Currently targetable oncogenic fusions are relatively rare in NSCLC; they are found in less than 1% to approximately 5% of tumors, depending on the fusion type and population. Nevertheless, the development of therapies targeting these oncogenic drivers has been associated with improved outcomes for molecularly selected subsets of patients with NSCLC, which remains the second most common cancer worldwide and the leading cause of cancer death.1,3-5
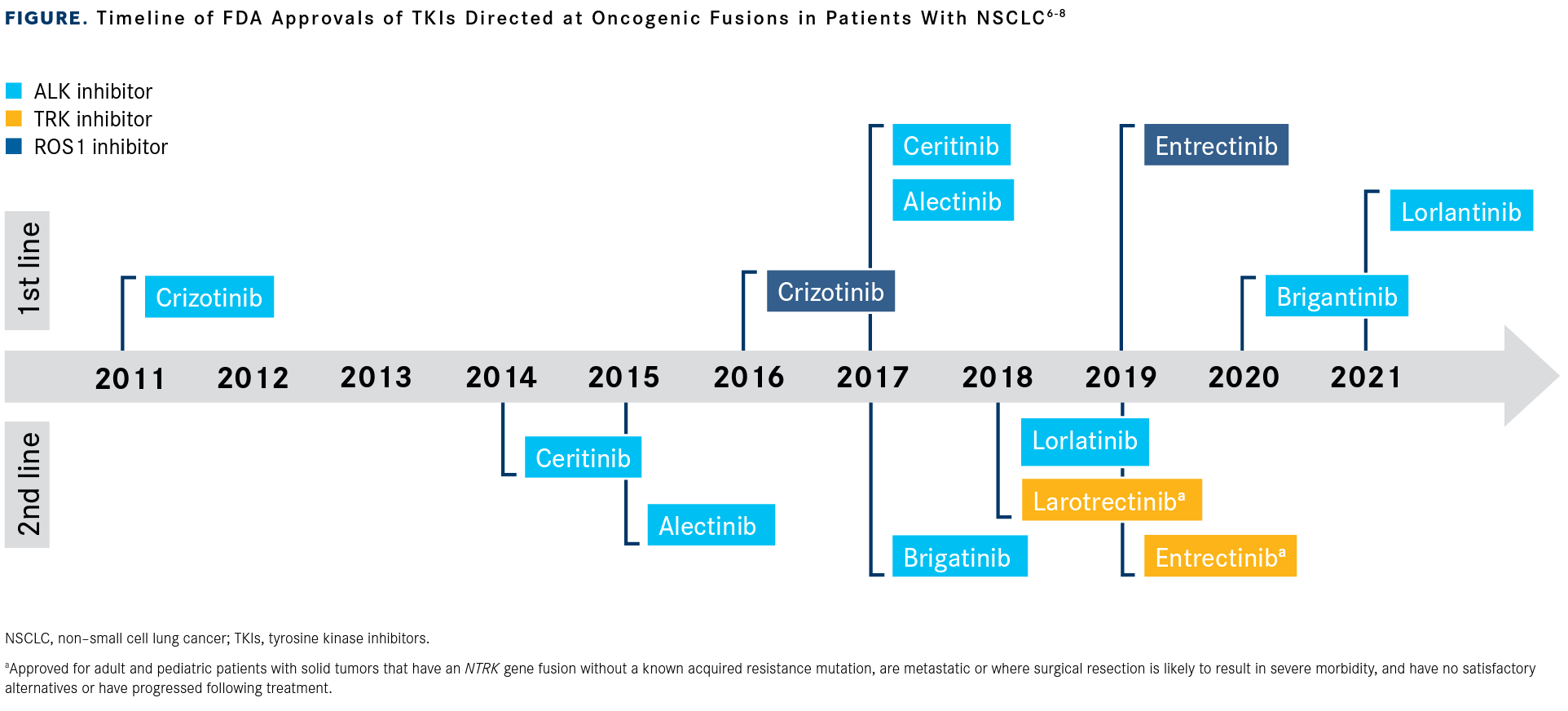
During a recent OncLive Peer Exchange®, a panel of lung cancer experts discussed advances in targeting oncogenic fusions in NSCLC, including NTRK gene fusions, ALK rearrangements, and ROS1 rearrangements. Starting in 2011, the FDA has approved therapies targeting these fusions in first- and second-line settings for populations that include patients with NSCLC (FIGURE).6-8
“We’re going to be entering a multidimensional universe of biomarkers for each patient. This isn’t going to be a binary world of EGFR mutant or wild-type, but it’s going to be secondary mutations, immunophenotypic markers that are complementing each other,” Martin F. Dietrich, MD, PhD, said.
The expansion of molecular markers shows the growing importance of next-generation sequencing, panelist Timothy Craig Allen, MD, JD, emphasized. “It is becoming impossible—if not already impossible—to find everything that’s out there just using [testing] kits or immunohistochemistry,” he said. “There is still, perhaps, a role for immunohistochemistry in [certain] settings, but I don’t think that excludes a full next-generation sequencing profile on our patients. We never know what we’re going to see and, as we know, even a small percentage of patients with lung cancer with a certain mutation, [considering] all the patients we have, there are going to be a reasonably large number of [patients] around the country who could benefit from [targeted] therapy.”
NTRK Fusions
“NTRK gene fusions encode TRK fusion proteins, which are oncogenic drivers in various tumor types,” Misako Nagasaka MD, PhD, explained. TRKs are expressed in normal human neuronal tissue, where they are activated by neurotrophins to facilitate the development and function of the human nervous system. Three transmembrane proteins encoded by 3 NTRK genes are known: TRKA by NTRK1, TRKB by NTRK2, and TRKC by NTRK3.9,10 NTRK gene fusions with a variety of partner genes have been observed in human cancers, which can result in an altered TRK fusion protein with enhanced kinase function that promotes cancer cell growth and survival. In NSCLC, NTRK gene fusions have been observed in less than 1% of tumors.3
Two FDA-approved tyrosine kinase inhibitors (TKIs) have shown significant benefit in targeting TRK fusion–positive cancers, including NSCLC: larotrectinib (Vitrakvi) and entrectinib (Rozlytrek).7,8 Entrectinib is also approved for ROS1-positive NSCLC.8
Larotrectinib
During the 2021 American Society of Clinical Oncology (ASCO) Annual Meeting, investigators presented an updated efficacy and safety analysis of the 3 pivotal larotrectinib trials, LOXO-TRK-14001 (NCT02122913), NAVIGATE (NCT02576431), and SCOUT (NCT02637687) that led to larotectinib’s approval.7,11 The analysis included data for 206 patients (median age, 38 years) with 21 different solid tumors.11
With a median follow-up of 22.3 months, the objective response rate (ORR) remained 75% (95% CI, 68%-81%), with a complete response (CR) in 22%, partial response (PR) in 53%, stable disease (SD) in 16%, and progressive disease (PD) in 6%. In 15 patients with brain metastases, the ORR was 73% (95% CI, 45%-92%), most of which were PRs (73%). There were no CRs. Among all patients, the median duration of response (DOR) was 49.3 months (95% CI, 27.3-not estimable [NE]). The median progression-free survival (PFS) was 35.4 months (95% CI 23.4-55.7), and the median overall survival (OS) was not reached; however, 77% of patients were alive at 36 months.
“Treatment-related adverse events [TRAEs] were mainly grade 1 and 2, with 18% having grade 3 or 4 TRAEs. Only 2% of patients discontinued treatment due to TRAEs,” Nagasaka said.
The benefit from larotrectinib therapy was maintained in extended follow-up data from an expanded population of 244 participants in the same pivotal studies with 25 different tumor types, including 9% with lung cancer, according to findings presented at the 2022 ASCO Annual Meeting. Among the expanded cohort, the ORR was 69% (95% CI, 63%-75%), with CRs in 21%, PRs in 43%, SD in 17%, and PD in 8%. Among 18 patients with baseline central nervous system (CNS) metastases, the ORR was 83% (95% CI, 59%-96%). After a median follow-up of at least 28.3 months, the median DOR for all evaluable patients was 32.9 months (95% CI, 27.3-41.7), the median PFS was 29.4 months (95% CI, 19.3-34.3), and median OS was not reached.12
Nagasaka noted that efficacy and safety findings for patients with NTRK fusion–positive lung cancer were comparable to data for the overall population, according to an analysis of participants in the NAVIGATE and LOXO-TRK-14001 trials.13 At 2022 ASCO, investigators presented updated findings for 26 patients with lung cancer included in those studies, including 24 who had adenocarcinoma histology, one with atypical carcinoid, and one with neuroendocrine histology.14 NTRK1 gene fusions were observed in 21 patients (81%) and NTRK3 fusions in 5 patients (19%).
Among 23 evaluable patients, larotrectinib therapy demonstrated an ORR of 83% (95% CI, 61%-95%), with 2 CRs, 17 PRs, 4 SD, and no PD. Of 10 evaluable patients with CNS metastases, the ORR was 80% (95% CI, 44%-97%), with no CRs, 8 PRs, 2 SD, and no PD. After follow-up of at least 12.9 months, the median for all evaluable patients was not reached for DOR (95% CI, 9.5-NE) or PFS (95% CI, 9.9-NE). The median OS was 40.7 months (95% CI, 19.4-NE).14
Most TRAEs were grade 1 or 2. Five patients reported grade 3 TRAEs of increased alanine and aspartate aminotransferase, myalgia, hypersensitivity, and weight gain. No patients discontinued treatment because of TRAEs.
Entrectinib
Investigators presented updated efficacy and safety data during the ASCO 2020 Virtual Scientific Program for patients treated with entrectinib during 3 phase 1/2 studies, ALKA372-001 (EudraCT 2012-000148-88), STARTRK-1 (NCT02097810), and STARTRK-2 (NCT02568267), whose findings paved the way for its FDA approval. The analysis included 74 evaluable patients with advanced or metastatic NTRK fusion–positive solid tumors, 13 of which were NSCLC.15
The ORR was 63.5% (95% CI, 51.5%74.4%) in all evaluable patients, 63.8% (95% CI, 50.1%-76.0%) in patients without baseline CNS disease, and 62.5% (95% CI, 35.4%-84.8%) in patients with baseline CNS disease. Among all evaluable patients, the median DOR was 12.9 months (95% CI, 9.3-NE), the median PFS was 11.2 months (95% CI, 8.0-15.7), and the median OS was 23.9 months (95% CI, 16.0-NE).
“Safety was in line with what was previously reported. The most common grade 3 or higher TRAEs were weight gain, anemia, and fatigue,” Nagasaka said. Weight gain and anemia both occurred in 7.1% of patients and fatigue in 6.2% of patients.
The latest data for a larger cohort of patients with NTRK fusion–positive solid tumors treated during the same clinical studies supported those findings, according to results presented at 2022 ASCO.16 Among 150 evaluable patients, the ORR was 61.3% (95% CI, 53.1%-69.2%), including CRs in 16.7%, PRs in 44.7%, SD in 10.0%, PD in 12.0%, missing or unevaluable scans for 12.0%. The median DOR was 20.0 months (95% CI, 13.2-31.1), the median PFS was 13.8 months (10.1-20.0), and the median OS was 37.1 months (95% CI, 27.2-NE).
Responses were observed for a range of tumor types. In 31 patients with NSCLC, the ORR was 64.5% (95% CI, 3.7-58.8). Among 31 patients with baseline CNS metastases across tumor types, the ORR also was 61.3% (95% CI, 42.2%-78.2%), including 2 patients with CRs, 17 with PRs, 4 with SD, and 2 with PD. The median DOR in the CNS cohort was 17.2 months (95% CI, 9.0-33.3), median PFS was 11.7 months (95% CI, 4.9-30.3), and median OS was 20.0 months (95% CI, 7.9-NE).
No new safety signals were observed in the safety population of 235 patients. TRAEs resulted in discontinuation in 7.2% of participants.
Treatment Selection and Sequencing
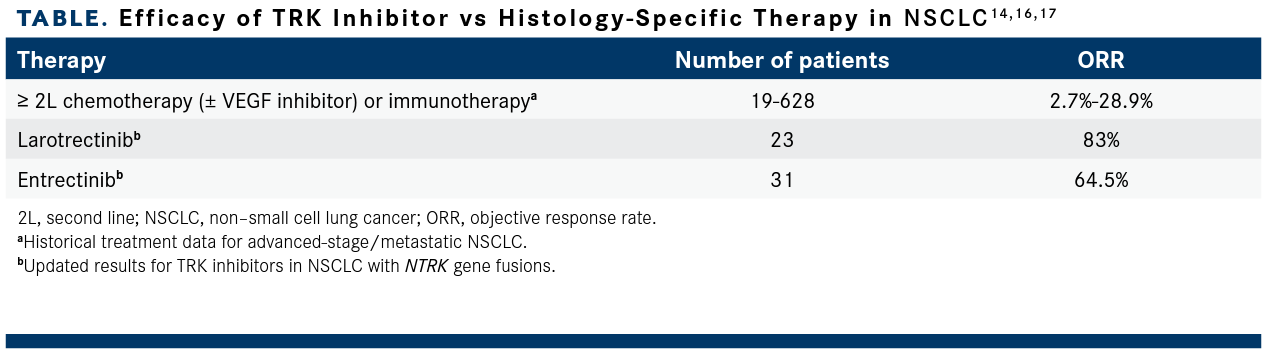
In a recently published study in The American Journal of Managed Care®, the authors stated that TRK inhibitors improve responses and survival for eligible patients vs historical outcomes with non–TRK-targeted standard therapies across most tumor histologies including in later lines of therapy where TRK inhibitors are likely to be used.17
In NSCLC, larotrectinib and entrectinib elicited a more robust response in patients with NTRK gene fusions than would be expected with standard therapies,17 a finding that persisted in updated data presented at 2022 ASCO (TABLE14,16,17).14,16 However, Carlson et al noted that “matching-adjusted indirect comparison and simulation studies suggest greater benefit to survival outcomes with larotrectinib compared with entrectinib.”17
Dietrich agreed with this assessment. “They’re both good drugs, but larotrectinib seems to be doing better in the lung space in both progression and OS. For familiarity, and because of those data pieces, that might be my preferred choice,” he said. Fernando C. Santini, MD, also agreed. “TKI therapies should be the first-line approach for all patients with NTRK fusion–positive cancer. Larotrectinib is probably a little more active and powerful. The response rates are amazing, along with the duration of response activity in the brain. It’s a no-brainer,” he said.
In patients experiencing progression on larotrectinib, Dietrich recommends rebiopsying and resequencing. “Many of these tumors remain dependent on the NTRK fusion,” he said, adding that several novel TRK inhibitors may offer promise in this setting, such as LOXO-195, repotrectinib, and taletrectinib. Of these, he said LOXO-195 is no longer in development but is still available on compassionate use through the manufacturer, whereas repotrectinib and taletrectinib are currently being explored in clinical trials.
Repotrectinib (TPX-0005) is a small macrocyclic TKI of ROS1, TRK, and ALK designed to overcome resistance mutations.18 In May 2022, the FDA granted repotrectinib a breakthrough therapy designation as a treatment for patients with ROS1-positive metastatic NSCLC who have previously been treated with a ROS1 TKI but have not received prior platinum-based chemotherapy. The drug previously gained breakthrough therapy status for 2 additional indications: patients with ROS1-positive metastatic NSCLC who have not been treated with a ROS1 TKI and patients with advanced solid tumors with an NTRK gene fusion who have progressed after 1 or 2 prior TRK TKIs, with or without prior chemotherapy, and have no satisfactory treatment options.18,19
Investigators are studying repotrectinib in the phase 1/2 TRIDENT-1 trial (NCT03093116) in patients with advanced solid tumors harboring ALK, ROS1, or NTRK1-3 rearrangements.20 Assessment of the early phase 2 TRIDENT-1 dataset that included the first 39 treated patients showed an ORR of 50% (3/6 patients; 95% CI, 12%-88%) among the NTRK-positive, TKI-pretreated patients.21 The ORR was 67% (4/6 patients; 95% CI, 22%-96%) among ROS1-positive patients previously treated with 1 prior TKI but no prior platinum-based chemotherapy, whereas it was 40% (2/5 patients each; 95% CI, 5%-85%) among both ROS1-positive patients pretreated with 1 prior TKI plus prior platinum-based chemotherapy and ROS1-positive patients pretreated with 2 prior TKIs but no prior platinum-based chemotherapy.
“There’s certainly some interest in establishing this second-line standard of care beyond its development as a ROS1 inhibitor,” Dietrich said. He noted this agent’s ability to overcome resistance mutations makes it particularly appealing in this setting vs the first-line setting, where Dietrich said larotrectinib is already “meeting all the hallmarks of brain efficacy, with the longterm durable responses that we would ask of a TKI in an oncogene-driven setting.”
Taletrectinib (AB-106; DS-6051b) is a next-generation TKI in development to target ROS1- and NTRK-positive lung cancer.22,23 It is currently being assessed as monotherapy in patients with ROS1 fusion-positive metastatic NSCLC and other solid tumors in the single-arm, multicohort the phase 2 TRUST-II study (NCT04919811). Mature data are not yet available.
ALK Rearrangements
ALK, which belongs to the insulin family of receptor tyrosine kinases, is usually expressed at low levels in regions of the CNS.24 In patients with solid tumors, ALK may be activated via a variety of mechanisms. In patients with NSCLC, ALK activation is usually the result of a chromosomal rearrangement in which the 3′ kinase domain of ALK becomes fused with various truncated portions of the EML4 gene and its associated promoter.24 ALK rearrangement has been reported in approximately 5% of NSCLC.4
“We’ve come a long way since the days of crizotinib [Xalkori],” Benjamin P. Levy, MD, who moderated the Peer Exchange program, said about the first ALK inhibitor to enter oncology practice. Following crizotinib’s FDA approval in August 2011, several other agents have been approved for the treatment of patients with ALK-positive metastatic NSCLC, including the second-generation TKIs alectinib (Alecensa), brigatinib (Alunbrig), and ceritinib (Zykadia), and the third-generation TKI lorlatinib (Lorbrena).6,25
Alectinib and Brigatinib
Updated OS and final PFS data for alectinib compared with crizotinib for treatment-naïve patients with ALK-positive NSCLC were reported from the phase 3 ALEX study (NCT02075840) in 2020.26 Final PFS data showed a median PFS of 34.8 months (95% CI, 17.7-NE) with alectinib vs 10.9 months (95% CI, 9.1-12.9) with crizotinib (stratified HR, 0.43; 95% CI, 0.32-0.58). OS data remained immature but with nearly 5 years of follow-up in the alectinib arm, the median OS was not reached with alectinib vs 57.4 months with crizotinib (stratified HR, 0.67; 95% CI, 0.460.98; P = .0376).
“The estimated 5-year OS rate for these patients is [approximately] 62%, which is remarkable; 43% of these patients will be event free in 4 years,” Santini said, referring to participants treated with alectinib. The OS benefit of alectinib was seen in patients with CNS involvement (HR, 0.58; 95% CI, 0.34-1.00) and those without CNS involvement (HR, 0.76; 95% CI, 0.451.26). The median treatment duration was 28.1 months with alectinib vs 10.8 months with crizotinib. No new safety signals were seen.
Updated OS and PFS data for ALK inhibitor–naïve patients with NSCLC treated with brigatinib during the phase 3 ALTA 1L (NCT02737501) trial were published in 2020.27 With a median follow-up of 24.9 months, the median PFS was 24.0 months (95% CI, 18.5-not reached) with brigatinib vs 11.0 months (95% CI, 9.2-12.9) with crizotinib (HR, 0.49; 95% CI, 0.350.68; P < .0001). Brigatinib also delayed median time to worsening of global health status/quality of life scores compared with crizotinib (HR, 0.70; 95% CI, 0.49-1.00; P = .049). Benefit was observed in patients with and without brain metastases. The median intracranial PFS in patients with brain metastases at baseline was 24.0 months (95% CI, 12.9-not reached) in the brigatinib arm vs 5.6 months (95% CI, 3.7-7.5) in the crizotinib arm (HR for intracranial progression or death, 0.31; 95% CI, 0.17-0.56; P < .0001).
“This shows that these are 2 very powerful second-generation TKIs with very good brain activity. Adverse effects are a little different, but they’re very safe drugs. In their final safety analyses, they didn’t show any new safety signals,” Santini said.
Lorlatinib
Compared with the second-generation agents alectinib and brigatinib, lorlatinib has been shown to be more potent in biochemical and cellular assays and to have the broadest coverage of ALK resistance mutations thus far identified.28 It was also designed to cross the blood-brain barrier to achieve high exposures in the CNS.27
In the phase 3 CROWN trial (NCT03052608), lorlatinib showed benefit over crizotinib.28 “In the intent-to-treat population, the time to CNS progression was significantly longer with lorlatinib than with crizotinib. The percentage of patients who were alive without CNS progression at 12 months was 96% with lorlatinib and 60% with crizotinib, with a hazard ratio of 0.07,” Nagasaka said.
Treatment Selection and Sequencing
When it comes to selecting which TKI to use in the first-line setting, Nagasaka said there are currently no clear answers because alectinib, brigatinib, and crizotinib have shown significant benefit, including in intracranial response rates. “In my practice, I’ve put patients on lorlatinib for first-line use, but I don’t put everyone on lorlatinib in the first line because it’s a little more difficult to manage than other drugs, like alectinib. Alectinib doesn’t have many adverse effects,” she said.
When it comes to lorlatinib’s AEs, Nagasaka said she doesn’t worry about hyperlipidemia or hypercholesterolemia, because those can be managed with statins and fenofibrates; however, she has concerns about lorlatinib’s cognitive effects. “I hesitate to start lorlatinib in patients who have a very demanding intellectual type of work or patients who are living alone and don’t have anyone to watch over them,” she said.
Nagasaka noted another important consideration in treatment decision-making is whether the patient has brain metastases at baseline. “There are potentially some data, although it’s preclinical, suggesting that perhaps in certain variants, like variant 3 [V3], we might need to take a more aggressive approach,” she said. In one study assessing outcomes in patients with NSCLC harboring a variety of EML4-ALK fusion variants, including V1, V2, and V3, those with V3 were found to have more metastatic sites at diagnosis vs those with V1 and V2; were more likely to experience earlier failure after treatment with first- and second-generation ALK TKIs, platinum-based combination chemotherapy, and cerebral radiotherapy; and had inferior OS.29
ROS1 Rearrangements
ROS1 rearrangements are observed in approximately 1% to 2% of patients with NSCLC.30 Although crizotinib is highly effective against ROS1-rearranged lung cancer, receiving FDA approval for this indication in the metastatic setting in 2016,31 it has limitations, including inadequate CNS drug penetration through the blood-brain barrier and the emergence of secondary kinase-domain mutations such as ROS1-G2032R.30,32 This has resulted in the search for agents that can overcome these limitations.
Entrectinib, which gained FDA approval for ROS1-positive metastatic NSCLC in 2019,8 has demonstrated a high, durable response rate. In an updated integrated analysis of the efficacy and safety of entrectinib in patients with locally advanced or metastatic ROS1 fusion–positive NSCLC (N = 161), the ORR was 67.1% (95% CI, 59.3%-74.3%).33 The median DOR was 15.7 months (95% CI, 13.9-28.6), the median PFS was 15.7 months (95% CI, 11.0-21.1), and the median OS was not estimable. Among 24 patients with measurable baseline CNS metastases, the intracranial ORR was 79.2% (95% CI, 57.9%-92.9%), the median intracranial PFS was 12.0 months (95% CI, 6.2-19.3), and the median intracranial DOR was 12.9 months (95% CI, 5.6-NE).
“In the ROS1 space, entrectinib has been particularly important, partly because of the fact that it’s CNS penetrant,” Hatim Husain, MD, said. “Before entrectinib, my practice was crizotinib. It’s an important medicine and it had some good clinical benefits for many patients. With the FDA approval of entrectinib, my practice pattern has changed to adopt entrectinib because of the CNS efficacy.”
The hope is that agents under investigation for ROS1 rearrangements, such as repotrectinib, taletrectinib, and brigatinib, will help overcome CNS penetration issues and resistance mutations such as G2032R. “Let’s see how all these medicines compete. The critical question is finding these patients, and that means next-generation sequencing, fusion-detection strategies around clinical suspicion—even with a negative result—and making sure patients are getting access, preferably before I/O [immuno-oncology] because of some of the toxicities that can be seen,” Husain said.
References
- Drusbosky LM, Rodriguez E, Dawar R, Ikpeazu CV. Therapeutic strategies in RET gene rearranged non-small cell lung cancer. J Hematol Oncol. 2021;14(1):50. doi:10.1186/s13045-021-01063-9
- Fusions in solid tumors. ESMO. February 1, 2018. Accessed June 4, 2022. https://bit.ly/3akeiJH
- Liu F, Wei Y, Zhang H, Jiang J, Zhang P, Chu Q. NTRK fusion in non-small cell lung cancer: diagnosis, therapy, and TRK inhibitor resistance. Front Oncol. 2022;12:864666. doi:10.3389/fonc.2022.864666
- Kim H, Chung JH. Overview of clinicopathologic features of ALK-rearranged lung adenocarcinoma and current diagnostic testing for ALK rearrangement. Transl Lung Cancer Res. 2015;4(2):149-155.doi:10.3978/j.issn.2218-6751.2014.12.02
- Mehta A, Saifi M, Batra U, Suryavanshi M, Gupta K.Incidence of ROS1-rearranged non-small-cell lung carcinoma in India and efficacy of crizotinib in lung adenocarcinoma patients. Lung Cancer (Auckl). 2020;11:19-25. doi:10.2147/LCTT.S244366
- Fukui T, Tachihara M, Nagano T, Kobayashi K. Review of therapeutic strategies for anaplastic lymphoma kinase-rearranged non-small cell lung cancer. Cancers (Basel). 2022;14(5):1184.doi:10.3390/cancers14051184
- FDA approves larotrectinib for solid tumors with NTRK gene fusions. FDA. December 14, 2018. Accessed June 4, 2022. https://bit.ly/3NWY5IK
- FDA approves entrectinib for NTRK solid tumors and ROS-1 NSCLC. FDA. August 16, 2019. Accessed June 4, 2022. https://bit.ly/392pvhW
- Amatu A, Sartore-Bianchi A, Siena S. NTRK gene fusions as novel targets of cancer therapy across multiple tumour types. ESMO Open. 2016;1(2):e000023. doi:10.1136/esmoopen-2015-000023
- Cocco E, Scaltriti M, Drilon A. NTRK fusion-positive cancers and TRK inhibitor therapy. Nat Rev Clin Oncol. 2018;15(12):731-747.doi:10.1038/s41571-018-0113-0
- Hong DS, Shen L, van Tilburg CM, et al. Long-term efficacy and safety of larotrectinib in an integrated dataset of patients with TRK fusion cancer. J Clin Oncol. 2021:39(suppl 15):3108. doi:10.1200/JCO.2021.39.15_suppl.3108
- Drilon AE, Hong DS, van Tilburg CM, et al. Long-term efficacy and safety of larotrectinib in a pooled analysis of patients with tropomyosin receptor kinase (TRK) fusion cancer. J Clin Oncol. 2022;40(suppl 16):3100. doi:10.1200/JCO.2022.40.16_suppl.310013
- Lin JJ, Kummar S, Tan D S-W, et al. Long-term efficacy and safety of larotrectinib in patients with TRK fusion-positive lung cancer. J Clin Oncol. 2021;39(suppl 15):9109. doi:10.1200/JCO.2021.39.15_suppl.9109
- Drilon AE, Lin JJ, Kummar S, et al. Updated efficacy and safety of larotrectinib in patients with tropomyosin receptor kinase (TRK) fusion lung cancer. J Clin Oncol. 2022;40(suppl 16):9024. doi:10.1200/JCO.2022.40.16_suppl.9024
- Rolfo CD, De Braud FG, Doebele RC, et al. Efficacy and safety of entrectinib in patients (pts) with NTRK-fusion positive (NTRK-fp) solid tumors: an updated integrated analysis. J Clin Oncol. 2020;38 (suppl 15):3605.doi:10.1200/JCO.2020.38.15_suppl.3605
- Krzakowski MJ,Lu S, Cousin S, et al.Updated analysis of the efficacy and safety of entrectinib in patients (pts) with locally advanced/metastatic NTRK fusion-positive (NTRK-fp) solid tumors. J Clin Oncol. 2022;40(suppl 16):3099. doi:10.1200/JCO.2022.40.16_suppl.3099
- Carlson JJ, Italiano A, Brose MS, et al. Comparative effectiveness of larotrectinib and entrectinib for TRK fusion cancer. Am J Manag Care. 2022;28(suppl 2):S26-S32. doi:10.37765/ajmc.2022.88845
- Repotrectinib. Turning Point Therapeutics. Accessed June 4, 2022.https://bit.ly/3NZ3xet
- Turning Point Therapeutics granted breakthrough therapy designation for repotrectinib treatment in patients with one prior ROS1 tyrosine kinase inhibitor and no prior chemotherapy. News release. Turning Point Therapeutics, Inc; May 10, 2022. Accessed June 4, 2022. https://yhoo.it/3NWmv5u
- A study of repotrectinib (TPX-0005) in patients with advanced solid tumors harboring ALK, ROS1, or NTRK1-3rearrangements (TRIDENT-1). ClinicalTrials.gov. Updated June 3, 2022. Accessed June 5, 2022. https://clinicaltrials.gov/ct2/show/NCT03093116
- Cho BC, Doebele RC, Lin J, et al. Phase 1/2 TRIDENT-1 study of repotrectinib in patients with ROS1+ or NTRK+ advanced solid tumors. J Thorac Surg. 2021;16(suppl 3):S174-S175.doi:10.1016/j.jtho.2021.01.251
- AnHeart Therapeutics announces first patient dosed in global phase 2 trust-II study for taletrectinib in ROS1 fusion-positive lung cancer. News release. AnHeart Therapeutics; October 26, 2021. Accessed June 5, 2022. https://www.anhearttherapeutics.com/news/press-releases/102621/
- Taletrectinib phase 2 global study in ROS1positive NSCLC (TRUST-II).ClinicalTrials.gov.Updated January 11, 2022. Accessed June 5, 2022. https://clinicaltrials.gov/ct2/show/NCT04919811
- Selinger CI, Rogers TM, Russell PA, et al. Testing for ALK rearrangement in lung adenocarcinoma: a multicenter comparison of immunohistochemistry and fluorescent in situ hybridization. Mod Pathol. 2013;26(12):1545-1553. doi:10.1038/modpathol.2013.87
- Cooper AJ, Sequist LV, Lin JJ. Third-generation EGFR and ALK inhibitors: mechanisms of resistance and management. Nat Rev Clin Oncol. Published online May 9, 2022.doi:10.1038/s41571-022-00639-9
- Mok T, Camidge DR, Gadgeel SM, et al. Updated overall survival and final progression-free survival data for patients with treatment-naive advanced ALK-positive non-small-cell lung cancer in the ALEX study. Ann Oncol. 2020;31(8):1056-1064.doi:10.1016/j.annonc.2020.04.478
- Camidge DR, Kim HR, Ahn MJ, et al. Brigatinib versus crizotinib in advanced ALK inhibitor-naive ALK-positive non-small cell lung cancer: second interim analysis of the phase III ALTA-1L trial. J Clin Oncol. 2020;38(31):3592-3603. doi:10.1200/JCO.20.00505
- Shaw AT, Bauer TM, de Marinis F, et al; CROWN Trial Investigators. First-line lorlatinib or crizotinib in advanced ALK-positive lung cancer. N Engl J Med. 2020;383(21):2018-2029.doi:10.1056/NEJMoa2027187
- Christopoulos P, Endris V, Bozorgmehr F, et al. EML4-ALK fusion variant V3 is a high-risk feature conferring accelerated metastatic spread, early treatment failure and worse overall survival in ALK+ non-small cell lung cancer. Int J Cancer. 2018;142(12):2589-2598. doi:10.1002/ijc.31275
- Katayama R, Gong B, Togashi N, et al. The new-generation selective ROS1/NTRK inhibitor DS-6051b overcomes crizotinib resistant ROS1-G2032R mutation in preclinical models. Nat Commun. 2019;10(1):3604.doi:10.1038/s41467-019-11496-z
- FDA expands use of Xalkori to treat rare form of advanced non-small cell lung cancer. News release. FDA; March 11, 2016. Accessed June 5, 2022. https://bit.ly/3Mlh7Yc
- Wrona A. Management of CNS disease in ALK-positive non-small cell lung cancer: is whole brain radiotherapy still needed?. Cancer Radiother. 2019;23(5):432-438.doi:10.1016/j.canrad.2019.03.009
- Dziadziuszko R, Krebs MG, De Braud F, et al. Updated integrated analysis of the efficacy and safety of entrectinib in locally advanced or metastatic ROS1 fusion-positive non-small-cell lung cancer. J Clin Oncol. 2021;39(11):1253-1263. doi:10.1200/JCO.20.03025









