Publication
Article
CAR T Pioneers Describe Challenges Faced in Cancer
Advances of chimeric antigen receptor T-cell therapy technologies are in rapid development and under investigation in a range of preclinical and clinical research around the globe.
Renier J. Brentjens, MD, PhD

Advances of chimeric antigen receptor (CAR) T-cell therapy technologies are in rapid development and under investigation in a range of preclinical and clinical research around the globe. In a paper recently published in Expert Review of Hematology, we reviewed the main challenges with current CD19-targeted CAR, obstacles to adopting CAR T-cell therapy in solid tumors, and various strategies that scientists are pursuing to overcome these issues.1
This update is the first in a 2-part series that provides an overview of antigen escape, T-cell persistence, safety when using CARs in hematologic malignancies within and beyond the setting of B-cell acute lymphoblastic leukemia (B-ALL), and the range of different strategies under investigation to address these issues.
Scientists at Memorial Sloan Kettering Cancer Center (MSK) pioneered CAR T-cell therapy as a treatment for cancer and were the first to demonstrate that CD19 was a robust target. We continue to explore new ways to advance CAR T cells as a treatment for other hematologic cancers, including lymphoma and multiple myeloma, as well as extending their use to solid tumors, including breast cancer, ovarian cancer, lung cancer, and mesothelioma.
The Evolution of CAR T-cell Therapy
CARs are genetically engineered receptors on T cells that include an HLA-independent antigen recognition domain, usually in the form of a single-chain Fv (scFv) from an antibody, combined with a T-cell activation domain.2 Once activated, CAR T cells become cytotoxic against the target antigen.
First-, second-, and third-generation CAR receptors have 0, 1, or 2 costimulatory domains, respectively. Newer generations, known as armored CARs, also deliver cytokines or PD-1–blocking scFvs, which enhance anticancer activity. Despite the high response rates of 80% to 90% achieved with CD19-targeted second-generation CAR T cells in treating B-ALL,3-8 they have not had comparable responses in other CD19-positive hematologic cancers or solid tumors.
Antigen Escape
An epitope is the specific segment of a protein to which the antibody binds. Data have demonstrated the target epitope loss in patients with B-ALL who lose durability of remission after treatment with CD19-directed CARs.9 Other research suggests that epitope loss may be secondary to deletions, mutations, and alternative splicing of the CD19 antigen recognition domain, allowing tumor cells to escape recognition.10
CD19 epitope loss has also led to the emergence of a myeloid clone after treatment for mixed-lineage leukemia- rearranged B-ALL in disease recurrence.11 Interestingly, antigen loss has also been observed in lymphoma12 and chronic lymphocytic leukemia13 with CAR T-cell therapy.
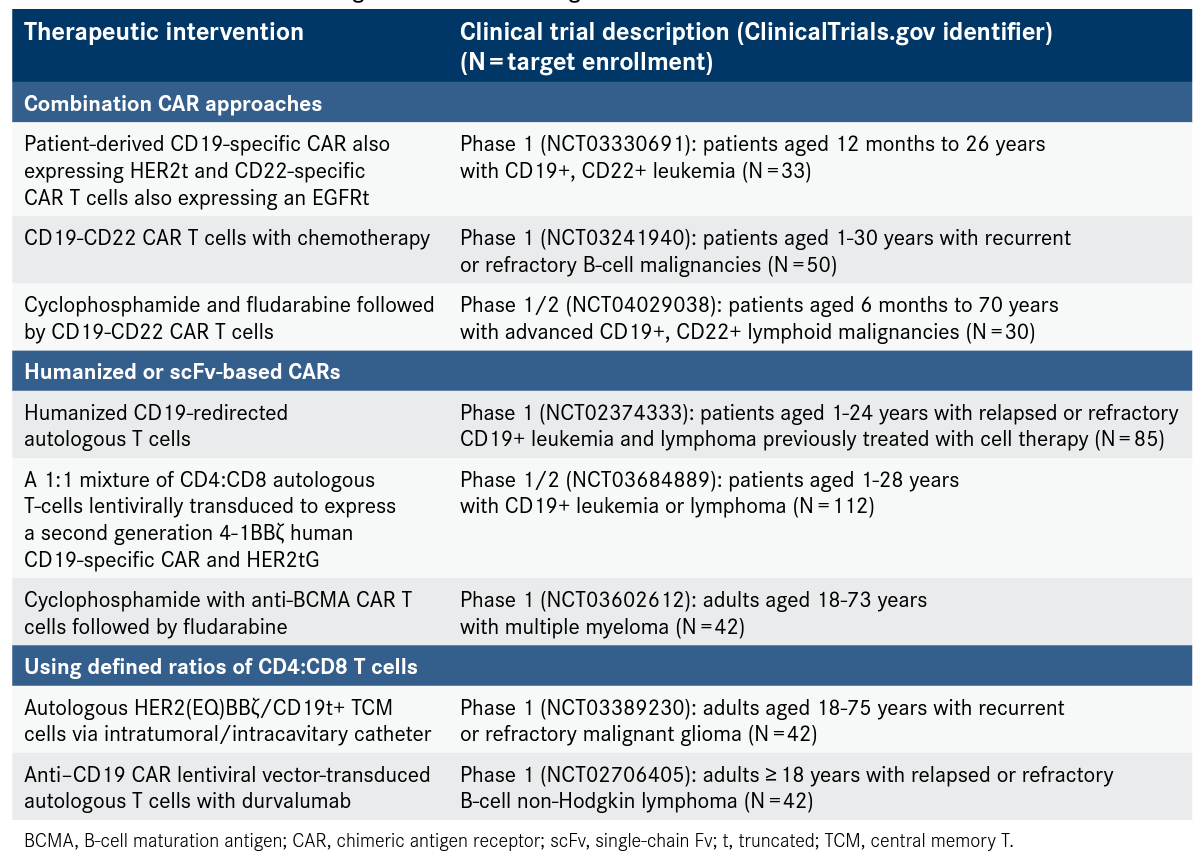
Preclinical models have shown that a process called trogocytosis may play a role in antigen escape with both CD28- and 4-1BB—based CARs. Trogocytosis is an active process that tumors use to transfer the target antigen onto T cells so they can escape destruction. Scientists believe this is reversible, but it has yet to be demonstrated in humans.14 One solution to overcome antigen escape is to combine single-target CARs that address different targets as a pooled product or use dual CARs15-17 or tandem CARs.18 Clinical trials are currently testing these combination approaches (Table). Another approach under investigation is low-dose radiation to sensitize antigen-negative tumor cells to CAR T cells and induce their death through the tumor necrosis factor—related apoptosis-inducing ligand.19
T-cell Persistence
The oncology community has yet to reach a consensus on the optimal duration of persistence for maintaining longterm remission with CAR T-cell therapy. Investigators have observed comparable response rates with a median duration of persistence of 14 days3 and 168 days.4 Still, relapses have been observed in some cases in which CARs were no longer performing immunosurveillance.8
T-cell stimulation is a double-edged sword: It increases persistence, but overstimulation can result in exhaustion.20 Some research has demonstrated that longevity can be improved without compromising efficacy by eliminating a signaling redundancy or by placing immunoreceptor tyrosine-based activation motifs near the membrane.21
A well-studied explanation for decreased persistence of CAR T cells is transgenic immune responses against scFv of murine origin.22-24 A fully human scFv-based CAR has shown promising activity against the target antigen in preclinical research.25 Human scFv-based CARs may be possible as a salvage therapy against the same antigen in patients treated with murine-scFv CARs, as demonstrated in a study among patients with B-ALL.26 Clinical trials using humanized as well as fully human scFv-based CARs are in progress (Table).
Several groups have studied central memory27,28 and stem cell memory T cells29-32 to learn more about persistence mechanisms mechanisms in adoptive cell therapy—for example, CARs. Approaches using defined ratios of CD4:CD8 T cells revealed that the CD4 subset is vital for boosting overall antitumor activity.33,34 Several clinical trials are ongoing in this area, including 1 study investigating humanized CARs and 2 studies using fully human scFv-based CARs (Table).
Investigators have achieved persistence and reduced exhaustion in CAR T cells by optimizing the ratio of CD4:CD8 cells through alterations to signaling domains and pathways.35 Another promising design modification involved diminishing redundant signaling, which improved effector and memory cell ratios and CAR persistence.21
An alternative approach to improving persistence involves stimulating the T-cell receptor using antigens from viruses, such as the Epstein-Barr virus or cytomegalovirus, and then using the cytotoxic T cells to transduce CARs. Current clinical trials testing this approach with hematologic and solid tumor antigen-directed CARs include NCT00085930, NCT01109095, NCT03768310, NCT01460901, and NCT01953900.
Finally, tumor vaccines and antigen-presenting cells may provide an immunological boost to CAR efficacy and persistence, and investigators are evaluating these in clinical trials NCT01953900, NCT02482532, NCT03186118.
Safety
One of the biggest challenges with CAR T-cell therapy is the potential for toxicity, as healthy cells may express the same targets as cancer cells. Cell engineers are incorporating “off” and “on” switches and employing other strategies to mitigate toxicity.
CARs with off switches, also called suicide genes, can be rapidly destroyed when toxicities arise or after they have eliminated cancer cells.36-39 Study data have recently shown that dasatinib (Sprycel) can pause CAR T-cell activity temporarily, acting as a reversible switch.40 Current trials investigating the safety of off switches include NCT02414269, NCT03696784, NCT03500991, NCT02311621, and NCT03016377. Other strategies for inactivating CARs involve checkpoint blockade,41 titrating activation with an antibody switch,42 and using self-limiting or transient CARs.43-46 On switches activate CARs only in the presence of an activating entity.47 A platform called GoCAR-T, in which a small molecule activates the costimulatory signal only when target antigen CAR interaction is present, is currently being tested in clinical trials. Recent data from a phase 1/2 study (NCT02744287) showed the approach effectively boosted expansion and persistence in 4 patients.48 The safety of the GoCAR-T platform may be further strengthened by adding an off switch.49 Another approach is to use synthetic Notch receptors that sense environmental cues and function as “smart” CARs.50
Toxicities associated with CAR T-cell therapy are cytokine release syndrome (CRS) and neurotoxicity, including temporary headache, confusion, and delirium. High-grade CRS, an acute inflammatory response characterized by fever and multiple organ failure and fever, can occur but is rare.
Beyond safety switches, scientists are investigating ways to reduce toxicities without compromising efficacy. For example, one strategy is the prophylactic administration of tocilizumab (Actemra), an IL-6—blocking antibody (NCT02906371), or an IL-1 antagonist.51 Emerging evidence in the setting of CD19-directed CARs for B-ALL shows that the severity of CRS depends on tumor burden.7,8,52 Therefore, strategies to reduce the risk of CRS include optimizing patient selection, changing the conditioning regimen,53 and using a split-dose approach54 for CAR T-cell infusion.
Advancing Innovation
At MSK, we are dedicated to finding new ways to improve patient outcomes through groundbreaking preclinical and clinical research. We are currently conducting 15 clinical trials testing CAR T-cell therapy in a range of indications, including lymphomas, multiple myeloma, advanced breast cancer, mesothelioma, and lung cancer.
Table. Select CAR Strategies Under Investigation
References
- Namuduri M, Brentjens RJ. Enhancing CAR T cell efficacy: the next step toward a clinical revolution? Expert Rev Hematol. 2020;13(5):533-543. doi:10.1080/17474086.2020.1753501
- Krause A, Guo HF, Latouche JB, Tan C, Cheung NK, Sadelain M. Antigen-dependent CD28 signaling selectively enhances survival and proliferation in genetically modified activated human primary T lymphocytes. J Exp Med. 1998;188(4):619-626. doi:10.1084/jem.188.4.619
- Park JH, Rivière I, Gonen M, et al. Long-term follow-up of CD19 CAR therapy in acute lymphoblastic leukemia. N Engl J Med. 2018;378(5):449-459. doi:10.1056/NEJMoa1709919
- Maude SL, Laetsch TW, Buechner J, et al. Tisagenlecleucel in children and young adults with B-cell lymphoblastic leukemia. N Engl J Med. 2018;378(5):439-448. doi:10.1056/NEJMoa1709866
- Davila ML, Rivière I, Wang X, et al. Efficacy and toxicity management of 19-28z CAR T cell therapy in B cell acute lymphoblastic leukemia. Sci Transl Med. 2014;6(224):224ra25. doi:10.1126/scitranslmed.3008226
- Grupp SA, Kalos M, Barrett D, et al. Chimeric antigen receptor-modified T cells for acute lymphoid leukemia. N Engl J Med. 2013;368(16):1509-1518. doi:10.1056/NEJMoa1215134
- Brentjens RJ, Davila ML, Rivière I, et al. CD19-targeted T cells rapidly induce molecular remissions in adults with chemotherapy-refractory acute lymphoblastic leukemia. Sci Transl Med. 2013;5(177):177ra38. doi:10.1126/scitranslmed.3005930
- Maude SL, Frey N, Shaw PA, et al. Chimeric antigen receptor T cells for sustained remissions in leukemia. N Engl J Med. 2014;371(16):1507-1517. doi:10.1056/NEJMoa1407222
- Grupp SA, Maude SL, Shaw P, et al. T cells engineered with a chimeric antigen receptor (CAR) targeting CD19 (CTL019) have long term persistence and induce durable remissions in children with relapsed, refractory ALL. Blood. 2014;124(21):380. doi:10.1182/blood.V124.21.380.380
- Sotillo E, Barrett DM, Black KL, et al. Convergence of acquired mutations and alternative splicing of CD19 enables resistance to CART-19 immunotherapy. Cancer Discov. 2015;5(12):1282-1295. doi:10.1158/2159-8290.CD-15-1020
- Gardner R, Wu D, Cherian S, et al. Acquisition of a CD19-negative myeloid phenotype allows immune escape of MLL-rearranged B-ALL from CD19 CAR-T-cell therapy. Blood. 2016;127(20):2406-2410. doi:10.1182/blood-2015-08-665547
- Brudno JN, Shi V, Stroncek D, et al. T cells expressing a novel fully-human anti-CD19 chimeric antigen receptor induce remissions of advanced lymphoma in a first-in-humans clinical trial. Blood. 2016;128(22):999. doi:10.1182/blood.V128.22.999.999
- Porter DL, Frey NV, Melenhorst JJ, et al. Randomized, phase II dose optimization study of chimeric antigen receptor modified T cells directed against CD19 (CTL019) in patients with relapsed, refractory CLL. Blood. 2014;124(21):1982. doi:10.1182/blood.V124.21.1982.1982
- Hamieh M, Dobrin A, Cabriolu A, et al. CAR T cell trogocytosis and cooperative killing regulate tumour antigen escape. Nature. 2019;568(7750):112-116. doi:10.1038/s41586-019-1054-1
- Qin H, Ramakrishna S, Nguyen S, et al. Preclinical development of bivalent chimeric antigen receptors targeting both CD19 and CD22. Mol Ther Oncolytics. 2018;11:127-137. doi:10.1016/j.omto.2018.10.006
- Ruella M, Barrett DM, Kenderian SS, et al. Dual CD19 and CD123 targeting prevents antigen-loss relapses after CD19-directed immunotherapies. J Clin Invest. 2016;126(10):3814-3826. doi:10.1172/JCI87366
- Hegde M, Corder A, Chow KK, et al. Combinational targeting offsets antigen escape and enhances effector functions of adoptively transferred T cells in glioblastoma. Mol Ther. 2013;21(11):2087-2101. doi:10.1038/mt.2013.185
- Hegde M, Mukherjee M, Grada Z, et al. Tandem CAR T cells targeting HER2 and IL13Rα2 mitigate tumor antigen escape. J Clin Invest. 2016;126(8):3036-3052. doi:10.1172.JCI83416
- DeSelm C, Palomba ML, Yahalom J, et al. Low-dose radiation conditioning enables CAR T cells to mitigate antigen escape. Mol Ther. 2018;26(11):2542-2552. doi:10.1016/j.ymthe.2018.09.008
- Wherry EJ, Kurachi M. Molecular and cellular insights into T cell exhaustion. Nat Rev Immunol. 2015;15(8):486-499. doi:10.1038/nri3862
- Feucht J, Sun J, Eyquem J, et al. Calibration of CAR activation potential directs alternative T cell fates and therapeutic potency. Nat Med. 2019;25(1):82-88. doi:10.1038/s41591-018-0290-5
- Maus MV, Haas AR, Beatty GL, et al. T cells expressing chimeric antigen receptors can cause anaphylaxis in humans. Cancer Immunol Res. 2013;1(1):26-31. doi:10.1158/2326-6066.CIR-13-0006
- Turtle CJ, Hanafi LA, Berger C, et al. Immunotherapy of non-Hodgkin’s lymphoma with a defined ratio of CD8+ and CD4+ CD19-specific chimeric antigen receptor-modified T cells. Sci Transl Med. 2016;8(355):355ra116. doi:10.1126/scitranslmed.aaf8621
- Turtle CJ, Hanafi LA, Berger C, et al. CD19 CAR-T cells of defined CD4+: CD8+ composition in adult B cell ALL patients. J Clin Invest. 2016;126(6):2123-2138. doi:10.1172/JCI85309
- Brudno JN, Hartman SD, Pittaluga S, et al. Clinical anti-lymphoma activity and toxicity of T cells expressing a novel anti-CD19 chimeric antigen receptor with fully-human variable regions. J Clin Oncol. 2018;36(suppl 15):3052. doi:10.1200/JCO.2018.36.15_suppl.3052
- Maude SL, Barrett DM, Rheingold SR, et al. Efficacy of humanized CD19-targeted chimeric antigen receptor (CAR)-modified T cells in children and young adults with relapsed/refractory acute lymphoblastic leukemia. Blood. 2016;128(22):217. doi:10.1182/blood.V128.22.217.217
- Wang X, Popplewell LL, Wagner JR, et al. Phase 1 studies of central memory-derived CD19 CAR T-cell therapy following autologous HSCT in patients with B-cell NHL. Blood. 2016;127(24):2980-2990. doi:10.1182/blood-2015-12-686725
- Yang S, Ji Y, Gattinoni L, et al. Modulating the differentiation status of ex vivo-cultured anti-tumor T cells using cytokine cocktails. Cancer Immunol Immunother. 2013;62(4):727-736. doi:10.1007/s00262-012-1378-2
- Gattinoni L, Lugli E, Ji Y, et al. A human memory T cell subset with stem cell-like properties. Nat Med. 2011;17(10):1290-1297. doi:10.1038/nm.2446
- Blaeschke F, Stenger D, Kaeuferle T, et al. Induction of a central memory and stem cell memory phenotype in functionally active CD4+ and CD8+ CAR T cells produced in an automated good manufacturing practice system for the treatment of CD19+ acute lymphoblastic leukemia. Cancer Immunol Immunother. 2018;67(7):1053-1066. doi:10.1007/s00262-018-2155-7
- Singh N, Perazzelli J, Grupp SA, Barrett DM. Early memory phenotypes drive T cell proliferation in patients with pediatric malignancies. Sci Transl Med. 2016;8(320):320ra3. doi:10.1126/scitranslmed.aad5222
- Sabatino M, Hu J, Sommariva M, et al. Generation of clinical-grade CD19-specific CAR-modified CD8+ memory stem cells for the treatment of human B-cell malignancies. Blood. 2016;128(4):519-528. doi:10.1182/blood-2015-11-683847
- Wang D, Aguilar B, Starr R, et al. Glioblastoma-targeted CD4+ CAR T cells mediate superior antitumor activity. JCI Insight. 2018;3(10):e99048. doi:10.1172/jci.insight.99048
- Sommermeyer D, Hudecek M, Kosasih PL, et al. Chimeric antigen receptor-modified T cells derived from defined CD8+ and CD4+ subsets confer superior antitumor reactivity in vivo. Leukemia. 2016;30(2):492-500. doi:10.1038/leu.2015.247
- Zhao Z, Condomines M, van der Stegen SJ, et al. Structural design of engineered costimulation determines tumor rejection kinetics and persistence of CAR T cells. Cancer Cell. 2015;28(4):415-428. doi:10.1016/j.ccell.2015.09.004
- Lupo-Stanghellini MT, Provasi E, Bondanza A, Ciceri F, Bordignon C, Bonini C. Clinical impact of suicide gene therapy in allogeneic hematopoietic stem cell transplantation. Hum Gene Ther. 2010;21(3):241-250. doi:10.1089/hum.2010.014
- Di Stasi A, Tey SK, Dotti G, et al. Inducible apoptosis as a safety switch for adoptive cell therapy. N Engl J Med. 2011;365(18):1673-1683. doi:10.1056/NEJMoa1106152
- Wang X, Chang W-C, Wong CW, et al. A transgene-encoded cell surface polypeptide for selection, in vivo tracking, and ablation of engineered cells. Blood. 2011;118(5):1255-1263. doi:10.1182/blood-2011-02-337360
- Vogler I, Newrzela S, Hartmann S, et al. An improved bicistronic CD20/tCD34 vector for efficient purification and in vivo depletion of gene-modified T cells for adoptive immunotherapy. Mol Ther. 2010;18(7):1330-1338. doi:10.1038/mt.2010.83
- Mestermann K, Giavridis T, Weber J, et al. The tyrosine kinase inhibitor dasatinib acts as a pharmacologic on/off switch for CAR T cells. Sci Transl Med. 2019;11(499):eaau5907. doi:10.1126/scitranslmed.aau5907
- Fedorov VD, Themeli M, Sadelain M. PD-1- and CTLA-4-based inhibitory chimeric antigen receptors (iCARs) divert off-target immunotherapy responses. Sci Transl Med. 2013;5(215):215ra172. doi:10.1126/scitranslmed.3006597
- Cao Y, Rodgers DT, Du J, et al. Design of switchable chimeric antigen receptor T cells targeting breast cancer. Angew Chem Int Ed Engl. 2016;55(26):7520-7524. doi:10.1002/anie.201601902
- Zhao Y, Zheng Z, Cohen CJ, et al. High-efficiency transfection of primary human and mouse T lymphocytes using RNA electroporation. Mol Ther. 2006;13(1):151-159. doi:10.1016/j.ymthe.2005.07.688
- Yoon SH, Lee JM, Cho HI, et al. Adoptive immunotherapy using human peripheral blood lymphocytes transferred with RNA encoding Her-2/neu-specific chimeric immune receptor in ovarian cancer xenograft model. Cancer Gene Ther. 2009;16(6):489-497. doi:10.1038/cgt.2008.98
- Beatty GL, Haas AR, Maus MV, et al. Mesothelin-specific chimeric antigen receptor mRNA-engineered T cells induce anti-tumor activity in solid malignancies. Cancer Immunol Res. 2014;2(2):112-120. doi:10.1158/2326-6066.CIR-13-0170
- Rowley J, Monie A, Hung C-F, Wu T-C. Expression of IL-15RA or an IL-15/IL-15RA fusion on CD8+ T cells modifies adoptively transferred T-cell function in cis. Eur J Immunol. 2009;39(2):491-506. doi:10.1002/eji.200838594
- Rodgers DT, Mazagova M, Hampton EN, et al. Switch-mediated activation and retargeting of CAR-T cells for B-cell malignancies. Proc Natl Acad Sci U S A. 2016;113(4):E459-E468. doi:10.1073/pnas.1524155113
- Taylor P. Bellicum reports first data on ‘controllable’ CAR-T. Fierce Biotech. December 14, 2018. Accessed June 29, 2020. https://www.fiercebiotech.com/biotech/bellicum-reports-first-data-controllable-car-t
- Duong MT, Collinson-Pautz MR, Morschl E, et al. Two-dimensional regulation of CAR-T cell therapy with orthogonal switches. Mol Ther Oncolytics. 2018;12:124-137. doi:10.1016/j.omto.2018.12.009
- Morsut L, Roybal KT, Xiong X, et al. Engineering customized cell sensing and response behaviors using synthetic notch receptors. Cell. 2016;164(4):780-791. doi:10.1016/j.cell.2016.01.012
- Giavridis T, van der Stegen SJC, Eyquem J, Hamieh M, Piersigilli A, Sadelain M. CAR T cell-induced cytokine release syndrome is mediated by macrophages and abated by IL-1 blockade. Nat Med. 2018;24(6):731-738. doi:10.1038/s41591-018-0041-7
- Park JH, Rivière I, Wang X, et al. Impact of disease burden on long-term outcome of 19-28z CAR modified T cells in adult patients with relapsed B-ALL. J Clin Oncol. 2016;34(suppl 15):7003. doi:10.1200/JCO.2016.34.15_suppl.7003
- Park JH, Rivière I, Wang X, et al. Impact of the conditioning chemotherapy on outcomes in adoptive T cell therapy: results from a phase I clinical trial of autologous CD19-targeted T cells for patients with relapsed CLL. Blood. 2012;120(21):1797. doi:10.1182/blood.V120.21.1797.1797
- Frey NV, Shaw PA, Hexner EO, et al. Optimizing chimeric antigen receptor (CAR) T cell therapy for adult patients with relapsed or refractory (r/r) acute lymphoblastic leukemia (ALL). J Clin Oncol. 2016;34(suppl 15; abstr 7002). doi:10.1200/JCO.2016.34.15_suppl.7002









