Publication
Article
Old Standards Are Revisited in HER2+ Early-Stage Breast Cancer
Author(s):
As targeted agents for various levels of HER2 expression have demonstrated dynamic interoperability and efficacy, the prognosis for HER2-positive disease has improved.
Virginia F. Borges, MD

As targeted agents for various levels of HER2 expression have demonstrated dynamic interoperability and efficacy, the prognosis for HER2-positive disease has improved. Because 15% to 20% of breast cancers are HER2-positive, investigators have carefully examined what makes each pathology unique and how best to address it.1,2
“HER2 is a gene that when turned on really drives how a breast cancer will behave,” Virginia F. Borges, MD, said in a recent OncLive Peer Exchange®. “Twenty years ago, women would often lose their lives to this type of cancer within 6 to 12 months. Now, with the advance of disease understanding and drugs that we’re going to be spending a lot of time talking about today, that’s been totally flipped on its head.”
Moderator Joyce A. O’Shaughnessy, MD, concurred, “It is absolutely remarkable, isn’t it, just in our lifetime we’ve gone from one of the worst possible cancers to really arguably the best [to treat] in terms of outcomes. Those days are well in the past.” During the discussion, the panel reviewed advances in early-stage and metastatic disease, including the latest standards of care and treatment sequencing changes.
Early-Stage Disease Standards Align With Patient Specificities
Debu Tripathy, MD, noted that although anthracycline-based regimens in HER2-positive disease are not common, there are scenarios in which neoadjuvant use of this antibiotic may be appropriate. “We tend to use a taxane and trastuzumab [Herceptin] or pertuzumab [Perjeta], for higher-risk disease,” he said. “The addition of platinum is also still controversial as we haven’t formally tested it, but in the metastatic setting the platinum doesn’t seem to add much.”
Tripathy added that he would consider keeping anthracycline-based therapy in patients with higher-risk disease “but will de-escalate and drop the carboplatin if there is resistance.” Additionally, if there is no response to neoadjuvant therapy, or if a patient has a biphenotypic tumor, he would do so as well, although that is not the usual practice. “If the lymph node is HER2-negative, but the primary tumor is HER2positive, those would be situations in which we would use anthracyclines.”
Preoperative therapy considerations for earlystage resectable disease require a more nuanced approach.3 In patients with stage I disease, “we proceed with surgery first to make sure that they are not upstaged, and then we use the [adjuvant paclitaxel and trastuzumab] APT regimen,” Tripathy explained.
Borges, agreed, adding that “in general, I am only holding off on preoperative therapy for those times when a patient is diagnosed early. For example, a screening mammogram just happened to catch it at the right moment, so you’re looking at tumor that is subcentimeter or even below 1.5. The below–0.5-to–2 zone starts to become the inflection point where I might lean in towards the neoadjuvant therapy,” she said.
In early-stage disease, O’Shaughnessy said, the pathology report influences her decisions. “There are few of those stage I patients out there that I’m just not ready to not give 2 chemotherapy agents to,” she said, citing how data from the FeDeriCa trial (NCT03493854) exploring the efficacy of subcutaneous pertuzumab and trastuzumab in a fixed-dose combination may impact practice.4
Maryam Lustberg, MD, MP, noted that in her experience, this regimen has demonstrated a notable effect. “The FeDeriCa [dose] was able to be implemented and was very well-tolerated and accepted by patients,” she said. “The primary advantage is that it absolutely reduces chair time and frees up our nursing colleagues to be able to take care of additional patients.”
All panel members said they use this regimen, with O’Shaughnessy elaborating: “The primary end point for the FeDeriCa trial was the trough levels of the pertuzumab, and a secondary end point was the trough level of the trastuzumab at cycle 8, and in both the pertuzumab and the trastuzumab, the subcutaneous trough levels were actually a little higher in the subcutaneous delivery than in the [intravenous]. There was no hint that even after 3 weeks we were losing the bioavailability and the persistence, so that was very reassuring.” She also noted that the pathologic complete response (pCR) was also equivocal at 60% for both arms.4 “You couldn’t get more identical and the disease-free survival data, which came later, were also spot-on.”
Adjuvant Treatment
Moving on to adjuvant considerations, the panel noted that ado-trastuzumab emtansine (T-DM1; Kadcyla) has become the standard of care for individuals with residual disease5 but that there are other treatment considerations depending on response to neoadjuvant therapy. “For our patients who receive their upfront neoadjuvant chemotherapy regardless of the regimen chosen, if they’ve achieved complete cell-killing in the tumor and lymph nodes, have the pCR, then we think that’s a fantastic situation and we would feel comfortable leaving them on the trastuzumab/pertuzumab for completion of the year of adjuvant therapy,” Borges said. “But then for the data that we saw in the KATHERINE trial [NCT01772472],6 which was practice-changing, we know that for those patients who don’t have pCR, their risk of developing metastatic disease is much more significant.”
The study evaluated trastuzumab vs T-DM1 in patients with HER2-positive breast cancer who have residual tumor in the breast or axillary lymph nodes following preoperative therapy.6 “There was about a 50% difference there, depending on the treatment that was administered in the adjuvant setting, and therefore the KATHERINE trial used T-DM1 in that space and showed that that would improve the cure rates, with approximately 12.5% of those patients potentially experiencing metastatic disease in the future,” Borges said. “So, for us that became practice-changing overnight, that anyone with residual disease would go on to receive the T-DM1.”
Overtreatment with preoperative and postoperative regimens has sparked interest in de-escalating regimens and led to such studies as the CompassHER2-pCR trial (NCT04266249),7 in which investigators are looking to use neoadjuvant docetaxel, trastuzumab, and pertuzumab followed by 1 year of trastuzumab and pertuzumab without additional chemotherapy after a pCR. “This does what I call Goldilocks it. Just right, not too little, not too much, knowing that you can always give more [therapy] after surgery if the patient doesn’t achieve a pCR and offer them T-DM1 with or without the addition of tucatinib [Tukysa], which is a tyrosine kinase inhibitor that specifically targets HER2,” Borges said.
Tucatinib received FDA approval in 2020 based on data from the HER2CLIMB trial (NCT02614794), in which the agent was administered to patients in the metastatic setting.8 In the adjuvant, early-stage setting, tucatinib is being investigated in the phase 3 CompassHER2 RD trial (NCT04457596) as a dual HER2 blockade option for those receiving T-DM1. Other antibody-drug conjugates under investigation include fam-trastuzumab deruxtecan-nxki (Enhertu), in DESTINY-Breast05 (NCT04622319) (Figure).9,10
Figure. Ongoing Trials in Relapse Prevention in High Risk HER2-Positive Breast Cancer9,10
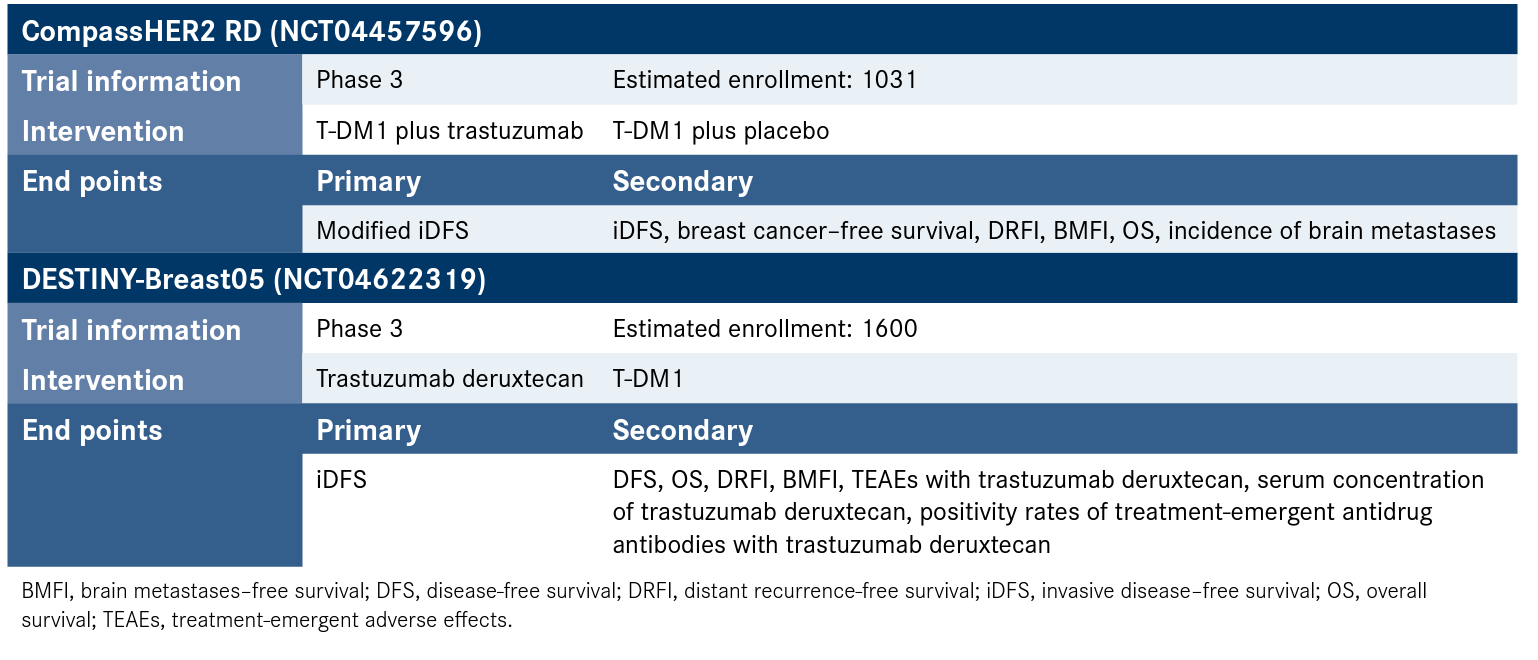
For now, T-DM1 remains the treatment of choice and current recommendation of the National Comprehensive Cancer Network (NCCN) for patients with residual/metastatic disease.5 “The very long preoperative chemotherapy regimen does a lot to patients, and the most common complaints that I’ve experienced with my patients are fatigue and neuropathy,” Lustberg said. “Initially, there was a question about whether T-DM1 could be safely combined with concurrent radiation therapy, and now we have a study that was reported out that it can. We are following that practice, with the concern being whether there would be increased rates of pneumonitis or interstitial lung disease, which were not seen with T-DM1.” Lustberg said that conveying expectations to patients is a key factor in delivering these treatments and that active symptom management also plays a role.
Tripathy pointed out that thrombocytopenia is also an adverse effect (AE) that should be monitored, but that neuropathy is more difficult for patients to cope with: “We monitor those patients closely, and a lot of them do drop off ahead of time.” O’Shaughnessy added that her experience with adjuvant T-DM1 has “largely has been very favorable. There are exceptions, [but] once they get a couple cycles, [patients] feel great. I’m impressed at the lack of fatigue and the fact that they can, pretty much, quickly get back into full life. The hair comes back, so the vast majority do well. However, I’ve had some patients go down on the dose because of thrombocytopenia or neuropathy. If it persists or worsens, I switch them back to the trastuzumab/pertuzumab, but they’re the distinct small minority and the vast majority do very well.”
Escalating Therapeutic Regimens
In the adjuvant setting for patients who received pertuzumab in addition to the backbone regimen of a taxane, carboplatin, and trastuzumab, there was an equivalent response to treatment with taxane, carboplatin, and trastuzumab alone in the phase 3 APHINITY trial (NCT01358877).11,12
At a preplanned 6-year analysis, a statistically significant difference was observed in invasive disease-free survival (iDFS) of 91% vs 88% with placebo (HR 0.76; 95% CI, 0.64 -0.91).12 The largest benefit was in the node-positive cohort, with a 5% difference in iDFS between the investigative and control study arms (HR, 0.72; 95% CI, 0.59-0.87), at 88% and 83%, respectively. “The node-negative population really didn’t seem to benefit,” O’Shaughnessy said. “Where I run into problems is T2 disease, maybe even 1.5 cm and above as an inflection point. Do you give them the [trastuzumab and chemotherapy] or you go with the APHINITY data [with the addition of pertuzumab] there?”
Tripathy said that he uses the FDA criteria of 2 cm or greater and that it is important to consider cost and adverse effects when weighing the small benefit provided by the addition of pertuzumab.13 “When you look at it more closely, cardiotoxicity is no different. There is more diarrhea, even in the maintenance phase there’s more diarrhea, but I still go by those criteria,” he said. “I discuss that with the patient. It’s important for them to know the small benefits and overwhelmingly, our patients really want to go ahead with the FDA 2-cm-cutoff. That’s what we use, but I agree with the numbers you provided, and they need to be shared with the patient and discussed.”
For patients who have had surgery first and have node-negative disease, Lustberg favors a dual HER2 approach: “I also see a bit of practice variation for patients who have had preoperative chemotherapy and achieve a pCR. If dual HER2-targeted therapy worked for them to help them achieve a pCR, it’s clear to me that you would continue that in the adjuvant setting. But I think perhaps it’s important to know that there is some practice variation across the country on this with some practitioners de-escalating to trastuzumab, especially in those who have node negativity.”
The prescribing information for pertuzumab in the neoadjuvant setting specifies that if dual blockade is given, it should be continued, even if patients achieve a pCR, O’Shaughnessy emphasized.13
“There have been data from the community setting that there’s been this tendency towards not continuing the pertuzumab in the setting of a pCR, which I would not do,” Borges added. “If I felt like the patient was high enough risk at the get-go to merit the combinatorial regimen, then that is what I’m committed to as the baseline, that’s what I’m going to carry through into the adjuvant setting even if they’ve achieved a pCR based on the data that we have today.”
The Role of Niraparib
Another potential escalation strategy is to use neratinib (Nerlynx), an oral pan-HER inhibitor tested in the adjuvant setting following trastuzumab in the ExteNET study (NCT00878709).14 “This study was [done] before we had pertuzumab and before T-DM1,” Tripathy noted. “[After] just the antecedent trastuzumab, the primary end point of iDFS was met. And it was, interestingly, very similar to the APHINITY, which also broadened the inhibition of the HER family which is, of course, what neratinib does as well.”
A more pronounced benefit was seen among those with estrogen receptor–positive disease. “It was 2.5% absolute improvement, but if you look at the subset analysis, all the benefit was in the ER–positive patients, where it was larger,” Tripathy said. “It went up to around 4.5% or so. And then they did an exploratory analysis of the 250 patients in the ExteNET who had gotten preoperative therapy. They still had residual disease and then they finished up their year of trastuzumab and were randomized to neratinib versus not. Which, of course, is how we treat the higher-risk patients. And there in the highest-risk population they saw the greatest benefit only in the ER-positive patients. But they had a 7.5% improvement in basic diseasefree survival and a surprising 9% absolute improvement in overall survival in the highest-risk population. And it was in those patients who were randomized within a year of having finished up their adjuvant trastuzumab.”
Tripathy said he reserves neratinib for higher-risk patients, generally those with residual disease or those who may have received adjuvant therapy but have node-positive disease. “I think it’s really interesting that we know the interaction of the HER2 and ER pathway to some extent, and I think that because HER signaling creates resistance to endocrine therapies, the blockade of that pathway may be making patients more sensitive to the endocrine therapy they’re getting,” he said. “That may be part of the benefit. But in any event, now that we have some methods to abate the diarrhea, which is really the biggest problem, by slow escalation of the dose, and I even go slower than what was published in the control study, and that has definitely helped me keep patients on treatment.”
Tripathy said he starts at 120 mg neratinib, escalating by the 40-mg tablet size. “I do it more slowly. I’ll go a whole week at the lower dose and then go up for another week. It may take me several weeks to get there, but I find that that’s a little bit better. Some patients are impatient, and they want to get up to the doses, so I just go with the control schema, which is a little more accelerated, as you know.”
Long-term Data
Long-term data from the APT trial (NCT00542451), in which adjuvant paclitaxel and trastuzumab were administered to patients with node-negative, HER2-positive breast cancer was presented at the 2022 San Antonio Breast Cancer Symposium. At the final, 10-year follow-up, analysis showed an iDFS rate of 91.3% (95% CI, 88.3%-94.4%) among 406 patients in the intention-to-treat population. The recurrence-free interval was 96.3% (95% CI, 94.3%-98.3%), and these results were consistent across patients with hormone receptor–positive (96.2%) and hormone receptor–negative (96.4%) disease.15
“At 5 years, the invasive disease-free survival was 96%, at 7 years it was 93%, and [at] the 10-year update, it’s down to 91%,” Tripathy said. “More hormone receptor–positive cancers, as you might expect, are having delayed recurrences. It’s still holding up to what the design was in terms of the event rate, but it is something that we need to track and maybe recalibrate who we might put on that study.”
Long-term follow-up was also presented from the ATEMPT trial (NCT01853748), in which patients received 1 year of adjuvant T-DM1 (n = 383) or trastuzumab/paclitaxel (n = 114).16 At a median follow-up of 5.8 months, the 5-year iDFS rate with T-DM1 was 97.0% (95% CI, 95.2%-98.7%), the 5-year recurrence-free interval was 98.3% (95% CI, 96.3%-99.0%), and the 5-year OS rate was 97.8% (95% CI, 96.3%-99.3%).
The T-DM1–alone regimen may actually be a better, but we don’t have the formal comparisons to be able to say that,” Tripathy observed. The study was not powered to evaluate the efficacy of trastuzumab/paclitaxel, but investigators reported that the 5-year iDFS was 91.3% (95% CI, 86.0%-96.9%).16
Another trial was initiated to compare these regimens, known as the ADAPT trial (NCT01817452).17 “This [was] really designed to compare the toxicities between the APT and the ATEMPT regimens, not to compare the outcomes,” Tripathy noted. “ATEMPT 2.0 [NCT04893109]18 is a larger study that is embarking. But again, the main objective there is to compare AEs. What we’re seeing preliminarily from the ATEMPT trial is that there is less long-term neuropathy, which is a problem you get [with] paclitaxel. Now there’s neuropathy, there’s thrombocytopenia, there’s higher discontinuation from treatment with T-DM1, but the longer-term neuropathy is actually less. There may, in the end, be some advantages from that that will be confirmed with the ATEMPT 2.0 trial.”
The clinical implications of these findings are already echoing in real-world practice.
“For the T-DM1 as adjuvant, I have used that for the patient who just digs in their heels and says, ‘I’m not going to do chemotherapy,’ ” Borges said. “And I realize what T-DM1 is as a drug but as a patient-facing option, it doesn’t quite feel the same as being asked to take something such as 12 weeks of paclitaxel or the regimen of [a taxane, carboplatin, trastuzumab]. Although it’s off guidelines, and I have a very careful conversation with the patient about that, I have in a few circumstances been able to get that approved. Or similarly, if I have a patient who has a cancer for which adjuvant therapy is going to be indicated but [she] has a lot of preexisting neuropathy, type 1 diabetes, for which a lot of steroids would be highly undesirable, [that] would be the other time I’ve turned to that as opposed to the no-treatment option that would be the alternative in those situations.”
Another consideration is the frequency of visits, especially if spikes in transmittable infections such as influenza or COVID-19 are reported. For instance, Lustberg said she used the ATEMPT regimen during COVID-19 surges. Additionally, drug-drug interactions play an important role. “There have been 2 patients who recently have had moderate interactions with their existing drugs and paclitaxel. For example, I have a patient who has a pretty severe preexisting seizure disorder and is on phenobarbital, and with paclitaxel, the interaction was moderate to severe, so I was able to get the 17 cycles of T-DM1 per ATEMPT approved and explained that ATEMPT 2.0 is looking at a much shorter duration of T-DM1. Depending on her toxicities, I will probably shorten off study.”
Patient profile also plays a role in the treatments Tripathy recommends. “I give the paclitaxel/trastuzumab or APT regimen for my patients with stage I, T1a, T1b, and even a few T1c disease for whom adjuvant treatment makes sense,” he said. “When a patient is T1a, you could say that gets into the territory of a no-adjuvant-treatment option. I treat a lot of very young women, aged 30 years, and they’re not comfortable with that. But I don’t want to subject them to the toxicities of [taxane, carboplatin, trastuzumab] or certainly not docetaxel, carboplatin, trastuzumab, and pertuzumab at that level. Trastuzumab/paclitaxel is a really nice comfort zone of being able to still give them something that I think is going to be effective against stage I disease, which some of these young women fall into a bit more than some of our older patients, although not exclusively.”
References
- Breast cancer HER2 status. American Cancer Society. Updated August 25, 2022. Accessed January 30, 2023. https://www.cancer.org/cancer/breast-cancer/understanding-a-breast-cancer-diagnosis/breast-cancer-her2-status.html
- Cancer stat facts: female breast cancer subtypes. National Cancer Institute Surveillance, Epidemiology, and End Results Program. Accessed January 30, 2023. https://seer.cancer.gov/statfacts/html/breast-subtypes.html
- Tolaney SM, Barry WT, Dang CT, et al. Adjuvant paclitaxel and trastuzumab for node-negative, HER2-positive breast cancer. N Engl J Med. 2015;372(2):134-141. doi:10.1056/NEJMoa1406281
- Tan AR, Im SA, Mattar A, et al; FeDeriCa study group. Fixed-dose combination of pertuzumab and trastuzumab for subcutaneous injection plus chemotherapy in HER2-positive early breast cancer (FeDeriCa): a randomised, open-label, multicentre, non-inferiority, phase 3 study. Lancet Oncol. 2021;22(1):85-97. doi:10.1016/S14702045(20)30536-2
- NCCN. Comprehensive Guidelines in Oncology. Breast cancer, version 1.2023. Accessed January 30, 2023. bit.ly/3X8bxxM
- von Minckwitz G, Huang CS, Mano MS, et al; KATHERINE Investigators. Trastuzumab emtansine for residual invasive HER2-positive breast cancer. N Engl J Med. 2019;380(7):617-628. doi:10.1056/NEJMoa1814017
- File D, Curigliano G, Carey LA. Escalating and de-escalating therapy for early-stage HER2-positive breast cancer. Am Soc Clin Oncol Educ Book. 2020;40:1-11. doi:10.1200/EDBK_100023
- FDA approves tucatinib for patients with HER2-positive metastatic breast cancer. FDA. Updated April 20, 2020. Accessed January 30, 2023. https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-tucatinib-patients-her2-positive-metastatic-breast-cancer
- T-DM1 and tucatinib compared with T-DM1 alone in preventing relapses in people with high risk HER2-positive breast cancer, the CompassHER2 RD trial. ClinicalTrials.gov. Updated February 17, 2022. Accessed January 30, 2023. https://clinicaltrials.gov/ct2/show/ NCT04457596
- A study of trastuzumab deruxtecan (T-DXd) versus trastuzumab emtansine (T-DM1) in high-risk HER2-positive participants with residual invasive breast cancer following neoadjuvant therapy (DESTINY-Breast05). Updated February 3, 2023. Accessed February 5, 2023. https://www. clinicaltrials.gov/ct2/show/NCT04622319
- Jagosky M, Tan AR. Combination of pertuzumab and trastuzumab in the treatment of HER2-positive early breast cancer: a review of the emerging clinical data. Breast Cancer (Dove Med Press). 2021;13:393407. doi:10.2147/BCTT.S176514
- Piccart M, Procter M, Fumagalli D, et al; APHINITY Steering Committee and Investigators. Adjuvant pertuzumab and trastuzumab in early HER2-positive breast cancer in the APHINITY trial: 6 years’ follow-up. J Clin Oncol. 2021;39(13):1448-1457. doi:10.1200/JCO.20.01204
- Phesgo. Prescribing information. Genentech, 2020. Accessed January 30, 2023. https://www.accessdata.fda.gov/drugsatfda_docs/label/2020/761170s000lbl.pdf
- Chan A, Moy B, Mansi J, et al; ExteNET Study Group. Final efficacy results of neratinib in HER2-positive hormone receptor-positive early-stage breast cancer from the phase III ExteNET trial. Clin Breast Cancer. 2021;21(1):80-91.e7. doi:10.1016/j.clbc.2020.09.014
- Taratino P, Tayob N, Dang CT, et al. Adjuvant trastuzumab emtansine versus paclitaxel plus trastuzumab for stage I HER2+ breast cancer: 5-year results and correlative analyses from ATEMPT (TBCRC033). Presented at: 2022 San Antonio Breast Cancer Symposium; December 6-10, 2022; San Antonio, TX.
- Tolaney S, Taratino P, Graham N, et al. Adjuvant paclitaxel and trastuzumab trial (APT) for node-negative, human epidermal growth factor receptor 2–positive (HER2+) breast cancer: final 10-year analysis. Presented at: 2022 San Antonio Breast Cancer Symposium; December 6-10, 2022; San Antonio, TX.
- A prospective, randomized multicenter, open-label comparison of preoperative combination of trastuzumab and pertuzumab with or without concurrent taxane chemotherapy given for twelve weeks in patients with operable HER2+/HR- breast cancer within the ADAPT protocol (ADAPT). ClinicalTrials.gov. Updated July 23, 2019. Accessed February 5, 2023. https://www.clinicaltrials.gov/ct2/show/NCT01817452
- ATEMPT 2.0: adjuvant T-DM1 vs TH. ClinicalTrials.gov. Updated January 31, 2023. Accessed February 5, 2023. https://clinicaltrials.gov/ct2/ show/NCT04893109








