News
Article
SABCS 2023 Findings Build on Positive Momentum in HER2+ Breast Cancer
Author(s):
Expert oncologists in the field of breast cancer review data in HER2-positive early-stage and metastatic breast cancer.
Joyce O’Shaughnessy, MD

The emergence of HER2-targeted therapies, such as trastuzumab (Herceptin), pertuzumab (Perjeta), ado-trastuzumab emtansine (Kadcyla), tucatinib (Tukysa), and fam-trastuzumab deruxtecan-nxki (Enhertu), in the past 25 years has afforded patients with HER2-positive breast cancer multiple effective treatment options, something that was practically nonexistent before the development of these agents. Now investigators are diligently working to determine which of these agents is suitable for a given patient in each disease setting in the HER2-positive space and developing new agents and combinations to further improve efficacy without increasing toxicity.
During a recent OncLive Peer Exchange, expert oncologists in the field of breast cancer discussed updated findings from clinical trials across HER2-positive early-stage breast cancer in the neoadjuvant and adjuvant settings. They also highlighted data from studies evaluating treatment options for patients with newly diagnosed and relapsed/refractory HER2-positive metastatic breast cancer. Results from the trials were presented during the 2023 San Antonio Breast Cancer Symposium (SABCS), which took place in December.
Neoadjuvant Treatment of HER2+ Early-Stage Breast Cancer
The panelists began their discussion by establishing the current treatment paradigm for patients with HER2-positive early-stage breast cancer in the neoadjuvant setting.
“If you reference the NCCN [National Comprehensive Cancer Network] guidelines, preoperative therapy is preferred for HER2-positive disease that is clinical [stage] T2 or greater or clinical N1 or greater,” Heather McArthur, MD, MPH, said. “Moreover, [the guidelines] recommend preoperative systemic therapy be considered for clinical T1c and N0 HER2-positive disease. It gives the data for all the potential preoperative regimens that you might expect, including the APHINITY [trial (NCT01358877)] regimen with anthracycline cyclophosphamide followed by a taxane with trastuzumab and pertuzumab.”
Building off established guidelines, there has been recent interest in using chemotherapy de-escalation in combination with HER2-targeted agents for patients with operable early-stage disease. During SABCS 2023, investigators presented findings from the phase 3 HELEN-006 trial (NCT00108745), which evaluated dual HER2 blockade with trastuzumab and pertuzumab in combination with de-escalated neoadjuvant nab-paclitaxel (Abraxane) monotherapy in patients with stage II to III HER2-positive, resectable early-stage breast cancer. Patients were randomly assigned 1:1 to receive trastuzumab plus pertuzumab in combination with either nab-paclitaxel (n = 332) or standard docetaxel plus carboplatin (n = 337).1
Findings from the study showed that patients in the nab-paclitaxel group experienced significantly higher pathological complete response (pCR) rates and improved tolerability compared with those in the docetaxel plus carboplatin arm. The pCR rates were 66.3% (95%, CI 61.2%-71.4%) vs 57.6% (95% CI, 52.3%-62.9%), respectively (OR, 1.448; 95% CI, 1.058-1.981; unadjusted P = .021). Grade 3 or 4 adverse effects (AEs) occurred at rates of 30.1% vs 38%, respectively (P = .032). Study authors concluded that their findings could reshape neoadjuvant therapy preferences in HER2-positive early-stage breast cancer.1
Adjuvant Treatment of HER2+ Early-Stage Breast Cancer
In 2012, the FDA approved the combination of pertuzumab with trastuzumab and docetaxel as the first dual anti-HER2 regimen for the treatment of patients with HER2-positive metastatic breast cancer. Then, in December 2017, the agency granted regular approval to pertuzumab plus trastuzumab and chemotherapy for use as adjuvant treatment for patients with HER2-positive early-stage breast cancer at high risk of recurrence.2
The regulatory decision was supported by findings from the phase 3 APHINITY trial, which compared adjuvant trastuzumab and chemotherapy in combination with either pertuzumab (n = 2400) or placebo (n = 2404). At the time of the approval, at a median follow-up of 45.4 months, patients in the investigational arm experienced a significant benefit in terms of invasive disease–free survival (iDFS) vs the control arm (HR, 0.82; 95% CI, 0.67-1.00; P = .047); the estimated 3-year iDFS rates were 94.1% (95% CI, 93.1%-95.0%) vs 93.2% (95% CI, 92.2%-94.3%), respectively.2
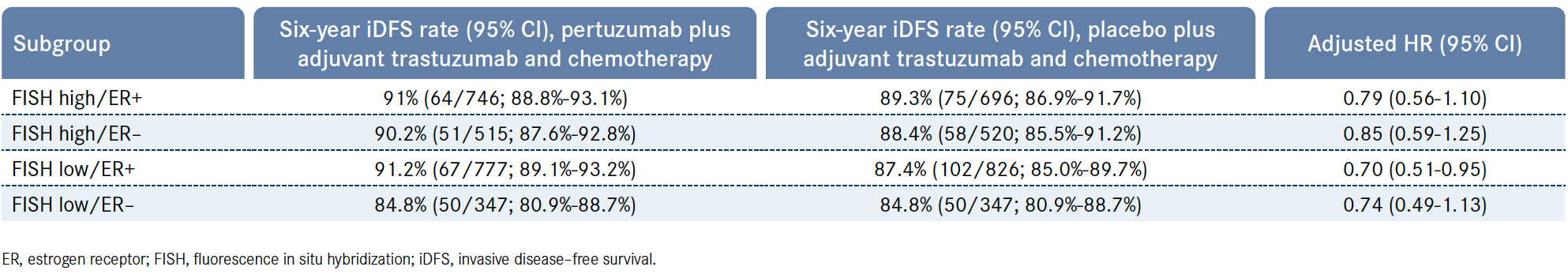
During SABCS 2023, investigators presented findings from an unplanned, exploratory analysis of APHINITY which sought to further define the benefit of adding pertuzumab to trastuzumab and chemotherapy according to a patient’s estrogen receptor (ER) and HER2-expression levels. HER2 fluorescence in situ hybridization (FISH) amplification ratio was defined as low (FISH ratio, 2 ≤ to < 5) vs high (≥ 5). ER expression per immunohistochemistry assessment was categorized as negative (< 1%) vs positive (≥ 1%).3
Findings from the analysis showed that patients who received pertuzumab experienced a similar IDFS benefit irrespective of ER or HER2 expression levels. Patients who were deemed to be FISH low/ER positive experienced the greatest IDFS benefit (HR, 0.70; 95% CI, 0.51-0.95), followed by those who were FISH low/ER negative (HR, 0.74; 95% CI, 0.49-1.13), FISH high/ER positive (HR, 0.79; 95% CI, 0.56-1.10), and FISH high/ER negative (HR, 0.85; 95% CI, 0.59-1.25; Table).3
Table. Six-Year iDFS Rates in Exploratory Analysis of Phase 3 APHINITY Trial3
Another trial that enrolled patients in the adjuvant setting that drew attention at SABCS 2023 with its positive findings was the phase 3 KATHERINE study (NCT01772472). Following treatment with neoadjuvant chemotherapy and HER2-targeted therapy, KATHERINE compared adjuvant treatment with trastuzumab emtansine vs trastuzumab in patients with HER2-positive early-stage breast cancer. The primary end point was IDFS; secondary end points included overall survival (OS) and safety.4
At a median follow-up of 8.4 years, data from the final IDFS and updated OS analysis presented at the meeting demonstrated that patients who received adjuvant trastuzumab emtansine (n = 743) experienced a 7-year iDFS rate of 80.8% vs 67.1% among patients who received adjuvant trastuzumab (n = 743). The HR for iDFS was 0.54 (95% CI, 0.44-0.66; P < .0001) in favor of trastuzumab emtansine. Moreover, the 7-year OS rates in the investigational vs the control arm were 89.1% vs 84.4%, respectively, and trastuzumab emtansine conferred a 34% reduction in the risk of death compared with trastuzumab (HR, 0.66; 95% CI, 0.51-0.87; P = .0027).4
Study authors noted in their conclusion that trastuzumab emtansine is the first treatment to display improved survival following surgery in patients with HER2-positive early breast cancer and residual disease after neoadjuvant therapy.4 “I very rarely get emotional during data presentations, but when those curves came up there [I thought], ‘Look at the separation.’ I was surprised. I didn’t think it was going to be that big,” Joyce O’Shaughnessy, MD, commented.
“We have excellent data from KATHERINE about what to do for the patients who haven’t achieved a pCR,” Claudine Isaacs, MD, said. “[However], there’s strong reason to enroll patients on these [other] existing trials to try and deal with the fact that [although] trastuzumab emtansine makes a huge difference, it still leaves 20% of patients having a recurrence and thinking about central nervous system [CNS] disease.”
First- and Second-Line Treatment of HER2+ Metastatic Breast Cancer
Transitioning their conversation to the treatment of patients with HER2-positive metastatic breast cancer, the panelists noted that the current standard of care in the frontline setting is also trastuzumab plus pertuzumab in combination with chemotherapy. Data from the final readout of the phase 3 CLEOPATRA study (NCT00567190) showed that patients with treatment-naive HER2-positive metastatic breast cancer who received docetaxel plus trastuzumab and pertuzumab (n = 402) experienced a median OS of 57.1 months (95% CI, 50-72) compared with 40.8 months (95% CI, 36-48) among patients who received placebo in place of pertuzumab (n = 406; HR, 0.69; 95% CI, 0.58-0.82).5
During SABCS 2023, investigators presented findings from the phase 1/2 ASPIRE trial (NCT03304080), which aimed to provide patients with hormone receptor–positive, HER2-positive metastatic breast cancer with a chemotherapy-free treatment option. The study evaluated the combination of anastrozole, palbociclib (Ibrance), trastuzumab, and pertuzumab in patients with previously untreated disease.
The coprimary end points were determining the maximum tolerated dose (MTD) and clinical benefit rate (CBR).6
Among patients treated at all dose levels (n = 30), the CBR was 97% (95% CI, 83%-100%). The objective response (ORR) was 73% (95% CI, 54%-88%), including a 4% CR rate. Safety data for the combination were consistent with the profiles of the individual agents, and the MTD of palbociclib as part of the regimen was determined to be 125 mg. Study authors noted that the combination could provide a viable chemotherapy- free alternative and further follow-up will determine the regimen’s impact on progression-free survival (PFS).6
For patients with active brain metastases, trastuzumab deruxtecan is being evaluated as a potential treatment option in the phase 2 TUXEDO-1 trial (NCT04752059). The study enrolled patients with HER2-positive breast cancer with brain metastases who underwent prior treatment with trastuzumab and pertuzumab. The primary end point was intracranial response rate; secondary end points included PFS, OS, and safety.7
Findings from the final outcome analysis of TUXEDO-1 presented during SABCS 2023 demonstrated that, at a median follow-up of 26.5 months, the median OS was not reached (NR; 95% CI, 22.2-NR) and the median PFS was 21 months (95% CI, 13.3-NR) among patients who received trastuzumab deruxtecan in the per-protocol population (n = 14). Study authors concluded that trastuzumab deruxtecan prolonged intra- and extracranial disease control with no new safety signals.7
Building off the efficacy seen with trastuzumab emtansine in patients with HER2-positive early-stage breast cancer, investigators evaluated the addition of tucatinib to the agent in the phase 3 HER2CLIMB-02 trial (NCT03975647). The study compared trastuzumab emtansine plus tucatinib or placebo in patients with HER2-positive locally advanced or metastatic breast cancer who had previously received trastuzumab and a taxane in any setting. The primary end point was PFS; secondary end points included OS and ORR.8
Updated findings from the study presented during SABCS 2023 showed that at the June 29, 2023, data cutoff, the median PFS in the combination arm (n = 228) was 9.5 months (95% CI, 7.4-10.9) compared with 7.4 months (95% CI, 5.6-8.1) in the placebo arm (HR, 0.76; 95% CI, 0.61-0.95; P = .0163). The combination also provided a PFS benefit over trastuzumab emtansine alone among patients with brain metastases (HR, 0.64; 95% CI, 0.46-0.89). The confirmed ORR was 42.0% vs 36.1%, respectively, with respective CR rates of 4.3% vs 4.2%.8
“Most trials allow patients who have had previously treated brain metastases, and trastuzumab deruxtecan and other antibody–drug conjugates [ADCs] have shown efficacy there,” Priyanka Sharma, MD, said. “But for patients who have active, untreated brain metastases or the ones [who] haven’t responded very well, right now the randomized data for tucatinib looks a lot more robust. For patients who have treated brain metastases, I am not always picking a tucatinib regimen in that second-line setting. The OS and PFS with trastuzumab deruxtecan is just so robust that I’m favoring that, but certainly for patients who have active metastasis tucatinib is a better option.”
Treatment of HER2+ Metastatic Breast Cancer in the Third Line and Beyond
The panelists closed out their discussion by briefly highlighting clinical trials examining treatment options for patients with HER2-positive metastatic breast cancer in the third line and later.
“When we get to fourth line, we’re in a tough situation where we have no guidelines,” Hope S. Rugo, MD, FASCO, said. “Obviously, what we want to do is go on to other novel therapies. Can you give ADC after ADC? Can you give new antibodies? Can you give different therapies? Otherwise, we’re really giving sequential chemotherapy based on what patients have seen before and responded to along with trastuzumab. So we’re reverting back to the old way, but [regarding] the new agents that are coming up, this is where we have a lot of excitement about how we could treat patients sequentially and maybe even move [these novel agents] earlier in some patients.”
During SABCS 2023, investigators presented primary results from a phase 2a study (NCT04224272) evaluating the triplet regimen of palbociclib, fulvestrant, and the novel bispecific antibody zanidatamab in patients with hormone receptor–positive, HER2-positive unresectable, locally advanced, and/or metastatic breast cancer. Patients needed to have undergone prior treatment with trastuzumab, pertuzumab, and trastuzumab emtansine to be eligible for the study. The primary end point of the single-arm study was safety in part 1 and 6-month PFS rate in part 2; secondary end points included ORR, OS, PFS, and safety.9
At the August 2023 data cutoff, patients who received the triplet (n = 51) experienced a median PFS of 12.0 months (95% CI, 8.0-15.0) and a 6-month PFS rate of 67.0% (95% CI, 52.0%-79.0%). The confirmed ORR was 35.0% (95% CI, 21.0%-50.0%), with a confirmed CR rate of 6.0%. The median duration of response was 15.0 months (95% CI, 12.0-25.0).9
Study authors concluded that the triplet showed a promising 6-month PFS rate and median PFS with a manageable safety profile. They argued that their findings supported further development of a novel chemotherapy-free regimen for patients with HER2-positive, hormone receptor–positive metastatic breast cancer who are heavily pretreated.9
References
- Liu Z, Chen X, Qiao J, et al. De-escalated neoadjuvant weekly nab-paclitaxel with trastuzumab and pertuzumab in HER2-positive early breast cancer (HELEN-006): a randomized, phase 3 trial. Abstract presented at: 2023 San Antonio Breast Cancer Symposium; December 5-9, 2023; San Antonio, TX. Abstract PO1-27-05.
- FDA grants regular approval to pertuzumab for adjuvant treatment of HER2-positive breast cancer. FDA. Updated December 21, 2017. Accessed January 8, 2024. https://www.fda.gov/drugs/resources-information-approved-drugs/fda-grants-regular-approval-pertuzumab-adjuvant-treatment-her2-positive-breast-cancer
- de Azambuja E, Agostinetto E, Samy F, et al. The benefit of adjuvant pertuzumab and trastuzumab according to estrogen receptor and HER2 expression: a sub-analysis of the APHINITY trial. Abstract presented at: 2023 San Antonio Breast Cancer Symposium; December 5-9, 2023; San Antonio, TX. Abstract PS09-04.
- Loibl S, Mano MS, Untch M, et al. Phase III study of adjuvant ado-trastuzumab emtansine vs trastuzumab for residual invasive HER2-positive early breast cancer after neoadjuvant chemotherapy and HER2-targeted therapy: KATHERINE final IDFS and updated OS analysis. Abstract presented at: 2023 San Antonio Breast Cancer Symposium; December 5-9, 2023; San Antonio, TX. Abstract GS03-12.
- Swain SM, Miles D, Kim SB, et al; CLEOPATRA Study Group. Pertuzumab, trastuzumab, and docetaxel for HER2-positive metastatic breast cancer (CLEOPATRA): end-of-study results from a double-blind, randomised, placebo-controlled, phase 3 study. Lancet Oncol. 2020;21(4):519-530. doi:10.1016/S1470-2045(19)30863-0
- Patel R, Cascetta K, Klein P, et al. A multicenter, phase I/II trial of anastrozole, palbociclib, trastuzumab, and pertuzumab in hormone receptor (HR)-positive, HER2-positive metastatic breast cancer (ASPIRE). Abstract presented at: 2023 San Antonio Breast Cancer Symposium; December 5-9, 2023; San Antonio, TX. Abstract RF02-01.
- Bartsch R, Berghoff AS, Furtner J, et al. Final outcome analysis from the phase II TUXEDO-1 trial of trastuzumab-deruxtecan in HER2-positive breast cancer patients with active brain metastases. Abstract presented at: 2023 San Antonio Breast Cancer Symposium; December 5-9, 2023; San Antonio, TX. Abstract PO2-04-05.
- Hurvitz SA, Loi S, O’Shaughnessy J, et al. HER2CLIMB-02: primary analysis of a randomized, double-blind phase 3 trial of tucatinib and trastuzumab emtansine for previously treated HER2-positive metastatic breast cancer. Abstract presented at: 2023 San Antonio Breast Cancer Symposium; December 5-9, 2023; San Antonio, TX. Abstract GS01-10.
- Escriva-de-Romani S, Cejalvo JM, Alba E, et al. Primary results from a phase 2a study of zanidatamab in combination with palbociclib plus fulvestrant in HER2+ HR+ metastatic breast cancer. Abstract presented at: 2023 San Antonio Breast Cancer Symposium; December 5-9, 2023; San Antonio, TX. Abstract LBO1-04.








