Publication
Article
Targeting FRα Enters a New Era in Ovarian Cancer and Beyond
Author(s):
Efforts to target the folate metabolism network have entered a new stage, with the approval of a novel therapy directed at folate receptor–α and the potential for additional agents aimed at that target in solid tumors.
Ursula A. Matulonis, MD

Folate metabolism has been successfully targeted in anticancer therapy for nearly 75 years, starting with the development of chemotherapeutic agents for treating hematologic malignancies. Now, efforts to leverage this network have entered a new stage, with the approval of a novel therapy directed at folate receptor–α (FRα) and the potential for additional agents aimed at that target in solid tumors.1,2
In November 2022, the FDA granted an accelerated approval for the use of mirvetuximab soravtansine-gynx (Elahere) to treat adults with FRα-positive, platinum-resistant epithelial ovarian, fallopian tube, or primary peritoneal cancer who have received 1 to 3 prior systemic regimens. The agency also approved the VENTANA FOLR1 RxDx Assay as a companion diagnostic.3
The approval is significant for patients with ovarian cancer for several reasons, according to Ursula A. Matulonis, MD, a coleader of the pivotal clinical trial that paved the way for the approval. Matulonis is chief of the Division of Gynecologic Oncology and the Brock-Wilson Family Chair at Dana-Farber Cancer Institute in Boston, Massachusetts.
Mirvetuximab soravtansine, an antibody-drug conjugate (ADC), is the first new therapy for platinum-resistant ovarian cancer since 2014, when a regimen containing bevacizumab (Avastin) was approved, Matulonis said in an interview with OncologyLive®. She also noted that the agent is one of the first biomarker-driven therapies for ovarian cancer, enabling screening of patients with recurrent disease at earlier stages.
Additionally, the approval helps generate opportunities for the development of other novel therapies for patients with ovarian cancer, including other ADCs, Matulonis noted. Overall, ADCs are “one of the fastest-growing therapeutic classes in oncology,” John W. Moroney, MD, said during a presentation at the 2022 American Society of Clinical Oncology (ASCO) Annual Meeting in June.4 Moroney is an associate professor of obstetrics and gynecology and associate professor of medicine at University of Chicago Medicine in Illinois.
Overall, the FDA has approved 13 ADCs since June 2019, including 6 for solid tumors and 7 for hematologic malignancies.3,5 One of those agents, belantamab mafodotin-blmf (Blenrep), is a multiple myeloma therapy that will be voluntarily withdrawn from the market after failing to meet efficacy requirements in a confirmatory study.6
In ovarian cancer alone, Moroney said, 10 ADCs are undergoing clinical testing in the United States for a range of targets including 3 directed at FRα: mirvetuximab soravtansine and 2 investigational therapies, farletuzumab ecteribulin (MORAb-202) and STRO-002. Meanwhile, several other strategies targeting FRα are being evaluated in ovarian cancer and other solid tumor types, according to information registered on ClinicalTrials.gov (Table).
Table. Select Clinical Trials Directed at Folate Receptor-α
Targeting the Folate Cycle
Folates are a family of B9 vitamins that are active in multiple components of complex cell metabolism and DNA synthesis and repair.7 Folates help mediate cell nutrients by converting amino acids such as serine and glycine to generate compounds such as purines and thymidylates.8,9
Although folates naturally occur in certain foods and can be synthesized in dietary supplements, cancer cells can hijack the folate metabolic cycle, an occurrence that has been recognized as one of the hallmarks of cancer. Investigators have explored interrupting oncogenic processes that occur through the folate cycle, including strategies that harness one of the 3 major mechanisms for the transport of folates through the cell membrane: the reduced folate carrier (RFC), the proton-coupled folate transporter (PCFT), and FRs. In 1948, methotrexate, imported into the cell primarily through RFC and PCFT, became the first antifolate. Other antifolates with RFC transport include pralatrexate and pemetrexed.1,8
The search for more selective therapies with fewer off-target toxicities has prompted investigators to attack dysregulated folate metabolism through FRα, a member of a high-affinity, folate-binding membrane glycoprotein family encoded by FOLR1. Other members of the family include FRβ, FRγ, and FRδ, encoded by FOLR2, FOLR3, and FOLR4, respectively.1,2
FRα is an attractive target for anticancer therapy because study findings have shown that it is overexpressed on the surface of malignant tissue in multiple solid tumor types whereas it is minimally or moderately detected in healthy tissue, typically in epithelial cells including those of the kidney, retina, choroid plexus, and placenta.2,7 Investigators have described a wide range of expression levels because of different techniques used to evaluate FRα. For example, FRα overexpression in non–small cell lung cancer was between 14% and 87%, depending on the study (Figure 1).2,10
Figure 1. FRα Overexpression in Diverse Tumor Types.2,10
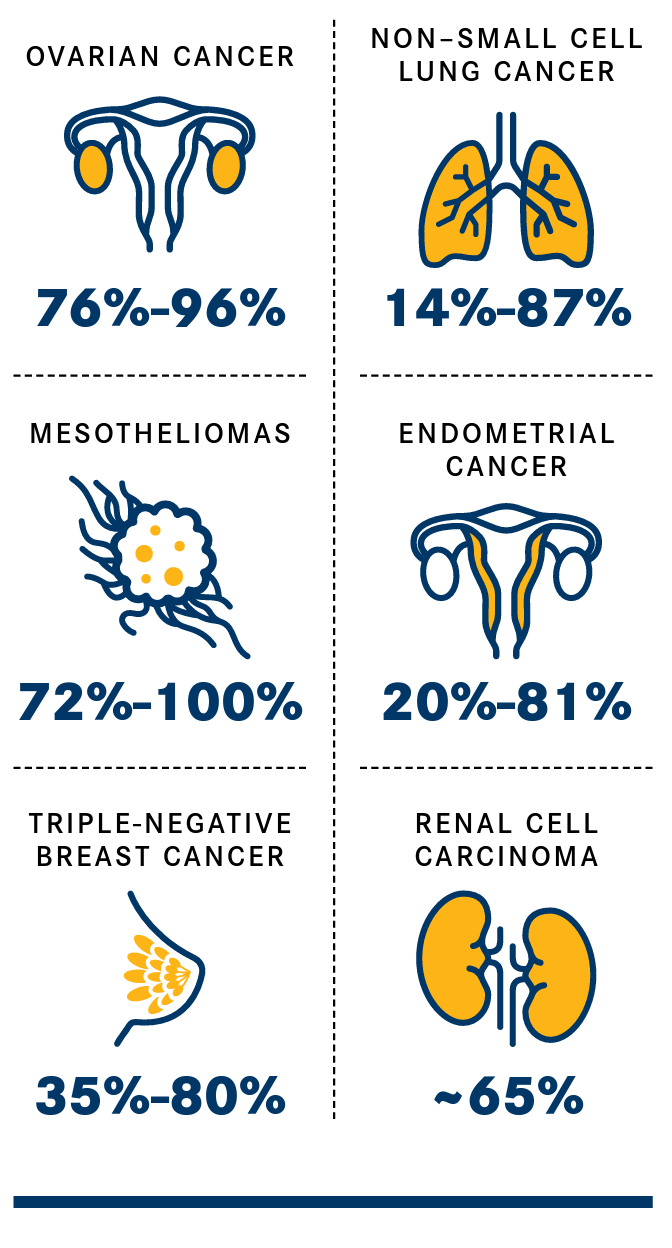
In ovarian cancer, FRα overexpression has been detected in nearly 90% of tissue samples, with the level varying by histological subtypes. FRα overexpression was 76% in high-grade serous tissue, 50% in low-grade serous samples, and 32% in clear cell carcinomas, according to a consortium-based analysis of data from 12 studies.2
Investigators have leveraged FRα expression to improve surgical outcomes in ovarian cancer. In November 2021, the FDA approved pafolacianine (Cytalux), a fluorescent drug that targets the FR, as an optical imaging agent to be used as an adjunct for intraoperative identification of malignant lesions.11
Pafolacianine was tested in a single-arm trial (NCT03180307) in patients with diagnosed or suspected ovarian cancer who were scheduled for primary surgical cytoreduction, interval debulking, or recurrent ovarian cancer surgery. Among 178 study participants, 26.9% had at least 1 evaluable ovarian cancer lesion detected with pafolacianine that was not found through standard visual or tactile inspection.11
FRα expression also is a benchmark for mirvetuximab soravtansine therapy. The definition of FRα positivity for patients who are candidates for treatment involves moderate (2+) or strong (3+) intensity levels (PS2+ scoringmethod) of 75% or more viable tumor cells with membrane staining in formalin-fixed, paraffin- embedded tissue using the VENTANA FOLR1 RxDx Assay. The immunohistochemistry (IHC) test is the only companion diagnostic approved for assessing FRα expression.7,12
Zeroing in on FRα
Initial efforts to target FRα in ovarian cancer stirred excitement among investigators but eventually faltered in late-stage clinical trials. Farletuzumab, a monoclonal antibody, and vintafolide, a small-molecule drug conjugate, failed to meet efficacy thresholds in studies conducted during the mid-2000s.10
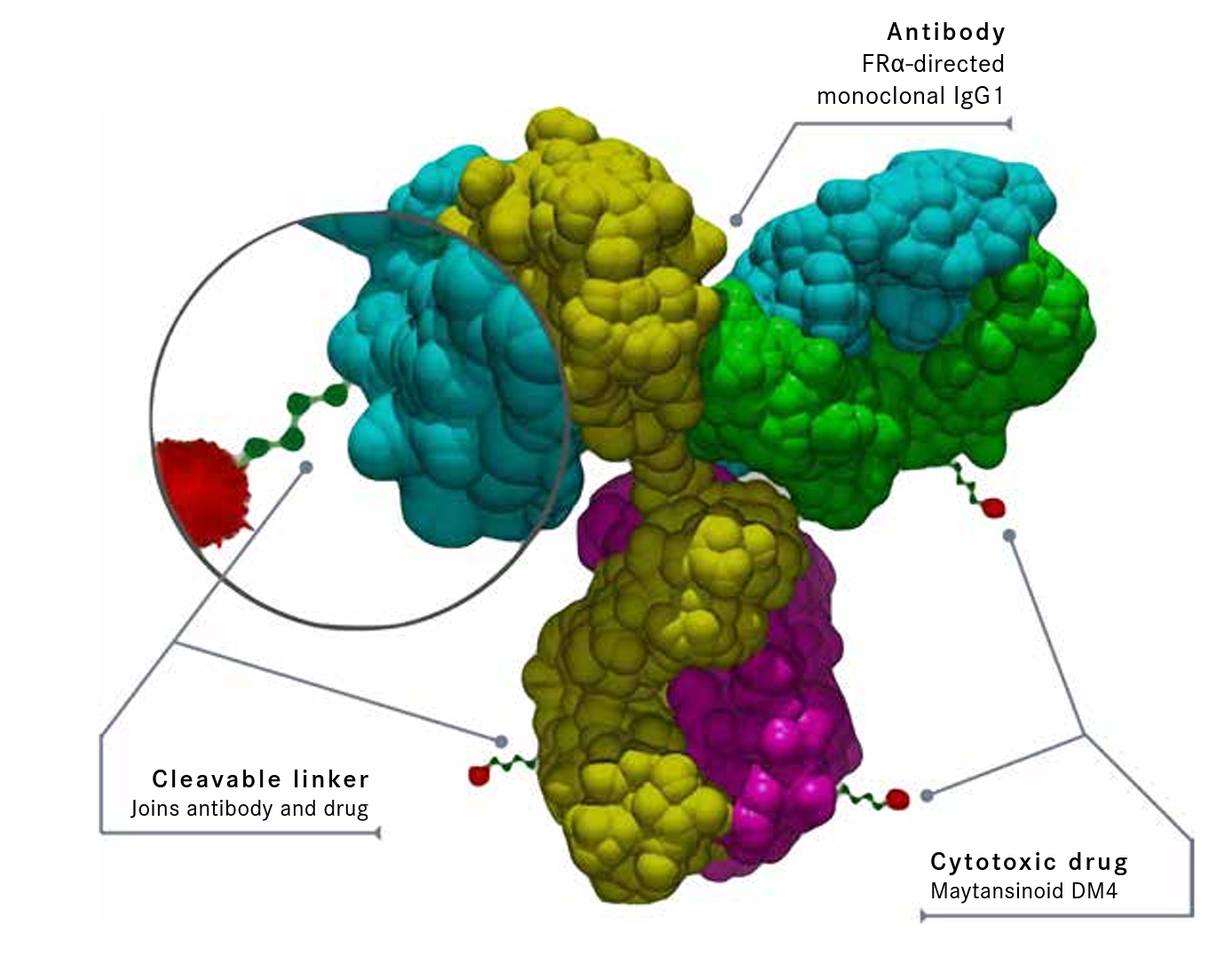
Mirvetuximab soravtansine is an ADC comprised of a humanized FRα–directed monoclonal IgG1 antibody linked to the maytansinoid DM4, a tubulin-targeting agent (Figure 2).10,13 Some investigators believe mirvetuximab soravtansine offers advantages over previous approaches because of the pairing of an antibody with a cytotoxic agent, the ability to target a specific antigen, and a linker that enables active DM4 metabolites to penetrate not only the target cells but also nearby tumor cells, creating a positive “bystander effect.”10
Figure 2. Key Components of Mirvetuximab Soravtansine10,13
Nevertheless, the journey of mirvetuximab soravtansine from novel concept to approved therapy has taken more than a decade, starting in 2011 when investigators began meeting with FDA officials to plan clinical trials of the agent. In June 2018, the FDA granted a fast-track designation for the therapy in patients with medium to high FRαpositive platinum-resistant epithelial ovarian cancer who had received up to 3 prior systemic regimens and who were candidates for singleagent chemotherapy as their next treatment.7
However, mirvetuximab soravtansine monotherapy did not demonstrate a statistically significant improvement in progression-free survival (PFS) over chemotherapy (paclitaxel, pegylated liposomal doxorubicin, or topotecan) in the phase 3 FORWARD I trial (NCT02631876). During the study, 366 patients were randomly assigned 2:1 to receive mirvetuximab soravtansine (n = 243) or chemotherapy (n = 109), with PFS in the intention-to-treat (ITT) and FRα-high populations as the primary end points.14
The HRs were 0.98 (95% CI, 0.73-1.31; P = .897) for the ITT group and 0.69 (95% CI, 0.48-1.00; P = .049) for participants with high FRα expression. Meanwhile, mirvetuximab soravtansine therapy resulted in superior outcomes in the FRα-high population over chemotherapy in terms of objective response rate (24% vs 10%, respectively) and patient-reported outcomes (27% vs 13%, respectively). There also were fewer treatment-related adverse events (AEs) of grade 3 or greater severity with mirvetuximab soravtansine than with chemotherapy (25.1% vs 44.0%, respectively) and fewer participants who discontinued therapy due to treatment-related AEs (4.5% vs 8.3%, respectively).14
Investigators were surprised by the negative PFS results. One potential reason that emerged from further analysis of the findings was that the method used for determining FRα positivity may not have been a reliable measure. Tumors were considered FRα positive by IHC with 50% or more of tumor cells with any membrane staining visible at 10 times or less microscopic objective magnification; 50% to 74% and 75% or more staining represented medium and high expression, respectively. By that threshold, 218 participants (59.6%) were deemed to have tumors with high FRα expression (≥ 75% staining).
By contrast, the scoring algorithm for the phase 1 development of mirvetuximab soravtansine took into consideration not only the proportion score of membranous staining by microscopic objective magnification but also the intensity of the staining. Under that method, only samples with PS2+ scores of 2+ and 3+ were labeled positive.14
Crossing the Finish Line
Following the FORWARD I results, representatives from ImmunoGen, Inc, the company developing mirvetuximab soravtansine, met with FDA officials to discuss whether data from a single-arm study could support an accelerated approval.7
In March 2020, the company launched the phase 3 SORAYA study (Study 0417; NCT04296890) in which mirvetuximab soravtansine was administered intravenously at 6 mg/kg (based on adjusted ideal body weight) every 3 weeks to patients with FRα-positive, platinum-resistant epithelial ovarian, fallopian tube, or primary peritoneal cancer. Participants could have received up to 3 prior lines of systemic therapy, with bevacizumab (Avastin) required.3,15 The PS2+ scoring method, as determined by the companion diagnostic, was used to define FRα positivity.
The FDA approved the drug based on overall response rate (ORR) and duration of response in 104 efficacy-evaluable patients. The median age was 62 years (range, 35-85). All patients had tumors with high-grade serous histology and 51% had visceral metastases at baseline. In terms of prior therapies, 89% had either 2 previous courses (39%) or 3 lines (50%). All patients had prior bevacizumab and 47% had received PARP inhibitor therapy.7
Overall, the confirmed ORR rate by investigator assessment was 31.7% (95% CI, 22.9%-41.6%), including a complete response rate of 4.8%. The median DOR was 6.9 months (95% CI, 5.6-9.7).13 These outcomes compare favorably with historical data for patients with ovarian cancer whose options for recurrent disease are limited to chemotherapy; the ORRs for these patients are approximately 12% to 13%, with median DORs of 4 months to 7.4 months.7
In terms of toxicities, the label for mirvetuximab soravtansine contains a boxed warning about the potential for severe ocular AEs, including visual impairment, keratopathy, dry eye, photophobia, eye pain, and uveitis. Overall, 61% of patients with ovarian cancer treated during SORAYA experienced ocular toxicities, including 9% of participants with grade 3 ocular AEs and 1 with grade 4 keratopathy. The median time to onset of the first ocular AE was 1.2 months (range, 0.03-12.9); 49% of affected patients had complete resolution of the AE and 39% had partial improvement at last follow-up.13
To mitigate the possibility of ocular AEs, patients should undergo an ophthalmic exam including visual acuity and slit lamp exam before starting mirvetuximab soravtansine, followed by testing every other cycle for the first 8 cycles and as clinically indicated. Premedication with ophthalmic topical steroids and the use of lubricating eye drops during treatment also are recommended.13
Other common all-grade AEs observed during SORAYA include fatigue (49%), nausea (40%), peripheral neuropathy (33%), and abdominal pain (36%). The incidence of grade 3/4 toxicities for these AEs was 3%, 0%, 2%, and 7%, respectively.13
Mirvetuximab soravtansine is undergoing further testing in the phase 3 MIRASOL study (Study 0416; NCT04209855), which the FDA has required as the confirmatory trial to support the accelerated approval. The study, which has a target enrollment of 453 participants, is comparing mirvetuximab soravtansine with investigator’s choice of chemotherapy (paclitaxel, pegylated liposomal doxorubicin, or topotecan) in patients with platinum-resistant, advanced highgrade epithelial ovarian, primary peritoneal, or fallopian tube cancers with high FRα expression.7 The trial is no longer recruiting participants.16
Future Directions in Targeting FRα
Besides mirvetuximab soravtansine, the development of novel therapies that target FRα in ovarian cancers and other solid tumors is continuing.
One of the most advanced in clinical development is farletuzumab ecteribulin, an ADC that was engineered after monotherapy results with farletuzumab proved disappointing. The humanized anti-FRα monoclonal antibody farletuzumab is conjugated to the cytotoxic microtubule inhibitor eribulin to form farletuzumab ecteribulin.4
The agent was tested in a phase 1 clinical trial (NCT03386942) in patients with FRα-positive solid tumors that was conducted in Japan. Among 24 patients in the ovarian cancer cohort who received farletuzumab ecteribulin intravenously at 0.9 mg/kg every 3 weeks, the ORR was 25.0% (95 % CI, 9.8%-46.7%). For 21 patients treated with the agent at 1.2 mg/kg every 3 weeks, the ORR was 52.4% (95% CI, 29.8%-74.3%). The ORR for patients with high-grade serous, platinum-resistant ovarian cancer for each dosing cohorts was 31.6% and 50.0%, respectively.17
FRα positivity was defined as greater than 5% of cells stained via IHC testing, with an intensity of 1+, 2+, or 3+. Antitumor activity was observed for tumors with FRα expression levels of less than 50% or less and at 50% or greater. Interstitial lung disease/pneumonitis was the most common treatment-related AE, affecting 37.5 % and 66.7% of patients with any-grade severity in the lower- and higher-dosing cohorts, respectively. However, only 1 instance of this AE was of grade 3/4 severity.17
Next steps for farletuzumab ecteribulin include plans for phase 2 trials in ovarian cancer (NCT05613088) and non–small cell lung cancer (NCT05577715).
STRO-002 is another novel FRα–targeting ADC that is being evaluated in solid tumors and in hematologic malignancies.
Investigators presented findings for STRO-002 in patients with a rare phenotype of acute myeloid leukemia (AML) at the 64th American Society of Hematology Annual Meeting and Exposition in December 2022. FRα is expressed in AML with the CBF2AT3-GLIS2 (CBF/GLIS) oncogenic fusion protein, described as “a highly refractory and uniformly fatal subtype of AML” that affects infants and young children.18
Among 16 patients with relapsed/refractory disease, 10 were treated with STRO-002 monotherapy, and 6 received the novel drug in combination with chemotherapy (fludarabine/cytarabine, decitabine, methotrexate, or dasatinib). Seven patients achieved a complete response, including 6 with negative minimum residual disease, and the remaining 9 patients had stable disease or a transient decrease in disease burden.18
References
- Gonen N, Assaraf YG. Antifolates in cancer therapy: structure, activity and mechanisms of drug resistance. Drug Resist Updat. 2012;15(4):183-210. doi:10.1016/j.drup.2012.07.002
- Scaranti M, Cojocaru E, Banerjee S, Banerji U. Exploiting the folate receptor αin oncology. Nat Rev Clin Oncol. 2020;17(6):349-359. doi:10.1038/s41571-020-0339-5
- FDA grants accelerated approval to mirvetuximabsoravtansine-gynx for FRα positive, platinum-resistant epithelial ovarian, fallopian tube, or peritoneal cancer. FDA. November 14, 2022. Accessed December 2, 2022. https://bit.ly/3VG8lJi
- Moroney JW. Beyond chemotherapy: antibody-drug conjugates in ovarian cancer. Presented at: 2022 ASCO Annual Meeting; June 3-7, 2022. Chicago, IL.https://bit.ly/3Fzfcze
- Pandit A. Antibody-drug conjugates in oncology: key considerations and future trends. Premier Research. October 13, 2022. Accessed December 6, 2022. https://bit.ly/3PasSnl
- GSK provides an update on Blenrep (belantamabmafodotin-blmf) US marketing authorisation. News release. GSK plc. November 22, 2022. Accessed December 6, 2022. https://bit.ly/3gZtEHr
- Multi-discipline review. Center for Drug Evaluation and Research. December 7, 2022. Accessed December 12, 2022. https://bit.ly/3UOStTZ
- Shuvalov O, Petukhov A, Daks A, Fedorova O, Vasileva E, Barlev NA. One-carbon metabolism and nucleotide biosynthesis as attractive targets for anticancer therapy. Oncotarget. 2017;8(14):23955-23977. doi:10.18632/oncotarget.15053
- Zarou MM, Vazquez A, Helgason GV. Folate metabolism: a re-emerging therapeutic target in haematological cancers. Leukemia. 2021;35(6):1539-1551. doi:10.1038/s41375-021-01189-2
- Birrer MJ, Betella I, Martin LP, Moore KN. Is targeting the folate receptor in ovarian cancer coming of age? Oncologist. 2019;24(4):425-429. doi:10.1634/theoncologist.2018-0459
- FDA approves pafolacianine for identifying malignant ovarian cancer lesions. FDA. Updated December 1, 2021. Accessed December 2, 2022. https://bit.ly/3UwH7nr
- List of cleared or approved companion diagnostic devices (in vitro and imaging tools). FDA. Updated December 2, 2022. Accessed December 12, 2022.https://bit.ly/3PiBzMC
- Elahere. Prescribing information. ImmunoGen, Inc; 2022. Accessed December 12, 2022. https://bit.ly/3W897z6
- Moore KN, Oza AM, Colombo N, et al. Phase III, randomized trial of mirvetuximabsoravtansine versus chemotherapy in patients with platinum-resistant ovarian cancer: primary analysis of FORWARD I. Ann Oncol. 2021;32(6):757-765. doi:10.1016/j.annonc.2021.02.017
- A study of mirvetuximabsoravtansine in platinum-resistant, advanced high-grade epithelial ovarian, primary peritoneal, or fallopian tube cancers with high folate receptor-alpha expression (SORAYA). ClinicalTrials.gov. Updated September 21, 2022. Accessed December 12, 2022. https://clinicaltrials.gov/ct2/show/NCT04296890?term=NCT04296890&draw=2&rank=1
- A study of mirvetuximabsoravtansine vs investigator’s choice of chemotherapy in platinum-resistant, advanced high-grade epithelial ovarian, primary peritoneal, or fallopian tube cancers with high folate receptor-alpha expression (MIRASOL). ClinicalTrials.gov. Updated October 31, 2022. Accessed December 12, 2022.https://clinicaltrials.gov/ct2/show/NCT04209855
- Nishio S, Yunokawa M, Matsumoto K, et al. Safety and efficacy of MORAb-202 in patients (pts) with platinum-resistant ovarian cancer (PROC): results from the expansion part of a phase 1 trial. J Clin Oncol. 2022;40(suppl 16):5513. doi:10.1200/JCO.2022.40.16_suppl.5513
- Meshinchi S, Miller L, MassaroS, et al.Anti-leukemic activity of STRO-002 a novel folate receptor-α (FR-α)-targeting ADC in relapsed/refractory CBF2AT3-GLIS2 AML. Blood. 2022;140(suppl 1):159-161. doi:10.1182/blood-2022-162842









