Gynecologic Oncology
Latest News

Latest Videos

CME Content
More News

In this seventh episode of OncChats: Immunotherapy and You, John Nakayama, MD, and Ali Amjad, MD, walk through a timeline of FDA approvals of immunotherapy approaches in gynecologic oncology and discuss associated toxicities to be aware of as well as how to manage them.

Dr Matulonis discusses the FDA approval of mirvetuximab soravtansine-gynx in folate receptor alpha–positive, platinum-resistant ovarian cancer, key efficacy and safety data from the SORAYA trial, and how further research can continue the momentum behind finding effective ovarian cancer treatments.

Dostarlimab plus standard-of-care chemotherapy, followed by dostarlimab alone, produced a statistically significant and clinically meaningful improvement in progression-free survival vs chemotherapy alone in patients with primary advanced or recurrent endometrial cancer.
Zimberelimab Monotherapy Elicits Responses in PD-L1–Positive Recurrent or Metastatic Cervical Cancer
Zimberelimab monotherapy demonstrated durable responses and was well tolerated in patients with PD-L1–positive, recurrent or metastatic cervical cancer who had progressed after first- or subsequent-line, platinum-containing chemotherapy, according to data from a phase 2 trial.

Nivolumab alone or in combination with ipilimumab produced encouraging responses that were maintained over time in patients with recurrent or metastatic cervical cancer.

In this sixth episode of OncChats: Immunotherapy and You, John Nakayama, MD, and Ali Amjad, MD, highlight the success seen with checkpoint inhibitors in gynecologic cancers, biomarkers of interest, and recent approvals in the space.
Kathleen Lutz, RN, NP-BC WH, explains the most commonly used first-line treatment options in patients with advanced endometrial cancer who are unsuitable candidates for surgery.
The panel reviews the current treatment modalities for endometrial cancer and in what circumstances each is considered for a patient.

Increasing the duration of bevacizumab treatment in the frontline setting did not improve progression-free survival for patients with advanced ovarian cancer.

PDS0101 given in conjunction with chemoradiation elicited an 100% overall response rate, along with tumor shrinkage greater than 60%, in 9 patients with high-risk, locally advanced cervical cancer, according to results of the phase 2 IMMUNOCERV trial.

Peter R. Dottino, MD, discusses the benefit of early-detection screening for endometrial cancer.
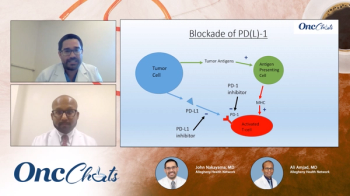
In this fifth episode of OncChats: Immunotherapy and You, John Nakayama, MD, and Ali Amjad, MD, discuss PD-1 blockade, potential biomarkers of response to treatment with immunotherapy, and the potential for these agents in the neoadjuvant setting.

Ritu Salani, MD, MBA, discusses the role of HER2 expression on treatment decisions in advanced uterine serous carcinomas.
The European Commission has approved cemiplimab-rwlc monotherapy for the treatment of adult patients with recurrent or metastatic cervical cancer who have progressed on or after platinum-based chemotherapy.

The panel shares how they determine a treatment regimen for patients with advanced endometrial cancer taking into consideration fertility and family planning, hereditary conditions, and comorbidities.

Kathleen Lutz, RN, NP-BC WH, presents a 76-year-old woman with advanced endometrial cancer, and the panel assesses the chosen treatment regimens.

Dr Crane discusses why Atrium Health decided to launch a gyn onc fellowship, how she helps fellows navigate the current political landscape while maintaining high-level care, and how she tries to model a good work-life balance for her trainees.

Onatasertib plus toripalimab elicited an objective response rate of 52.4% in patients with relapsed or metastatic cervical cancer, irrespective of PD-L1 expression.
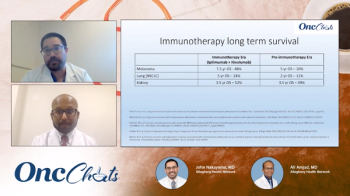
In this fourth episode of OncChats: Immunotherapy and You, John Nakayama, MD, and Ali Amjad, MD, highlight long-term survival data reported with immunotherapy approaches in patients with gynecologic cancers.

Pratibha Binder, MD, discusses how the introduction of novel immune checkpoint inhibitors into frontline treatment strategies could ameliorate unmet needs in endometrial cancer.

Ritu Salani, MD, MBA, discusses updates in treating endometrial cancer.

Ursula A. Matulonis, MD, discusses the significance of the FDA approval of mirvetuximab soravtansine-gynx in patients with folate receptor α–positive, platinum-resistant ovarian cancer.

The FDA has granted accelerated approval to mirvetuximab soravtansine-gynx (Elahere) for the treatment of select patients with folate receptor α–positive, platinum-resistant epithelial ovarian, fallopian tube, or primary peritoneal cancer.

Cheryl Saenz, MD, discusses the need for ongoing research efforts in cervical cancer.

At the request of the FDA, GlaxoSmithKline plc will restrict the second-line maintenance indication for niraparib to only the population of patients with recurrent ovarian cancer whose tumors harbor deleterious or suspected deleterious germline BRCA mutations.