Publication
Article
Oncology & Biotech News
Crisis Averted? Manufacturers Scurry to Meet Demand for Drugs
Author(s):
The shortage, which began in 2009, accelerated in 2010, and has shown no signs of abatement so far in 2011, according to Erin R. Fox, PharmD, manager of the Drug Information Service at University of Utah Health Care
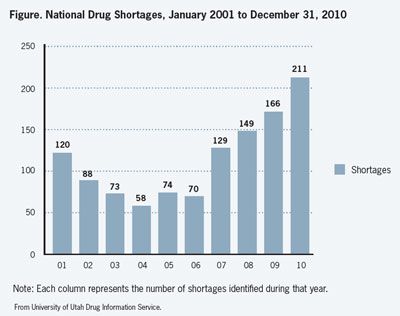
The shortage, which began in 2009, accelerated in 2010, and has shown no signs of abatement so far in 2011, according to Erin R. Fox, PharmD, manager of the Drug Information Service at University of Utah Health Care, which provides data for the American Society of Health-System Pharmacists (ASHP) Drug Shortage Resource Center. Dr Fox has led the drug shortages project since 2001.
“Through December 2010 we’ve tracked 211 new shortages, and that’s the most ever,” Fox said. This compares with 149 new shortages in 2008 and 166 in 2009 (Figure), according to the Drug Information Service’s data.
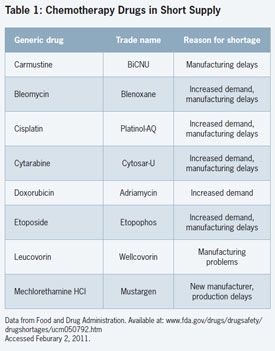
While the shortages include a variety of drugs, such as antimicrobials and anesthetic agents, the FDA reports that about 75% involve chemotherapy sterile injectables for cancer patients.
“Already in 2011 we have seen recalls due to quality issues and have seen new shortages occur as a result,” said Captain Valerie Jensen, RPh, the FDA’s Associate Director of the Drug Shortage Program.
In explaining one of the key reasons for the shortage, Fox, who is also associate professor (adjunct) in the Department of Pharmacotherapy at the University of Utah College of Pharmacy in Salt Lake City, added, “What makes chemotherapy drugs so different to deal with is basically there are no alternatives and they’re not easy to substitute. It’s a disaster.”
The shortages of chemotherapy agents (Table) are “definitely affecting patient care,” according to Bona E. Benjamin, BS Pharm, Director of the Medication-Use Quality Improvement for the practice development division of the ASHP.
“Regarding oncology drug shortages, we’ve gotten anecdotal reports that some patients are not getting drugs when they should be, that they’re not getting all of the drugs they need, or are not getting any drugs at all for a time,” Benjamin said. “And that means patients are not getting the best outcome that they should.”
Her contentions about patient care are corroborated by Ali McBride, PharmD, MS, a clinical pharmacy specialist at the Alvin J. Siteman Cancer Center at Barnes-Jewish Hospital, in St. Louis, Missouri. “We’re seeing a lot of shortages,” McBride said. His unit has delayed patients starting chemotherapy until they were able to get an emergency supply of a drug. In some cases, he said, autologous transplants also have been delayed.
One example he gave concerns the shortage of intravenous (IV) etoposide, a chemotherapeutic agent used in a variety of cancers, including lung cancer. In response to the shortage, some physicians have been switching from an IV to an oral formulation. “This increases the risk of dosage errors because patients usually have to double up on the oral medication,” McBride said. “This is truly a crisis, when patients can’t get the treatment regimen that they need,” he said.
In an attempt to find solutions for the shortages, ASHP, the Institute for Safe Medication Practices (ISMP), the American Society of Anesthesiologists, and the American Society of Clinical Oncology convened a Drug Shortages Summit in Bethesda, Maryland, in November 2010.
The summit found that a major cause of the shortages is that fewer manufacturers are producing the sterile injectables, most of which are generic, thereby limiting contributors to the supply chain. Consequently, manufacturing and production-line problems result in stunted supply. The problem of few suppliers came sharply to light when two of the largest sterile injectable manufacturers, Teva Pharmaceuticals and Hospira Inc., had to shut down some production lines in 2010 because of difficulty in meeting required quality control standards.
“Not many people are manufacturing (the target drugs) these days. There’s just not enough resiliency in the supply chain,” Fox said.
Benjamin concurred. “It’s very complex to produce sterile injectables, especially if you’re talking about cytotoxic drugs such as chemotherapy agents,” she said.
Asked to comment on the FDA’s understanding of the shortages, Captain Jensen said:
“In general, the older sterile injectable drugs are being made by fewer and fewer manufacturers each year. The firms continuing to make these older injectables have limited capacity and only so many products can be made on the existing manufacturing lines. The firms often discontinue these older drugs in favor of newer, more profitable drugs. Unfortunately, when a quality problem develops at one manufacturer or there is any delay or a discontinuation by a manufacturer, a shortage usually develops due to the limited ability of the remaining firms to meet the shortfall. The recent shortages have mainly been caused by a large number of quality issues, as well as delays and discontinuations.”
An FDA survey concluded that 43% of the shortages of sterile injectables in 2010 was attributable to product quality issues, such as particulate and microbial contamination, newly identified impurities, and chemical stability changes. Another 18% of the shortages was caused by manufacturers simply discontinuing production of a drug and 9% was due to raw material issues.
The point about raw materials is cogent when one considers that an estimated 80% of the raw materials used in the production of pharmaceuticals comes from outside the US, according to an ASHP report published in 2009. The reliance on foreign raw materials, the report said, can result in supply problems at home when war or political upheaval disrupts trade, when disease-causing microbes contaminate the extraction process, or when climatic or environmental conditions retard growth of plants used to make the raw materials.
The summit report says that suppliers’ struggles to comply with good manufacturing practices have been exacerbated by increased FDA inspections of injectable products. The FDA has increased the inspections, the report noted, “based on the higher likelihood of harm” to patients if the manufacturing processes are inconsistent with stated quality standards.
In addition to fewer suppliers, more stringent production standards, and harder-toget raw materials, rising worldwide demand for injectable chemotherapeutic drugs is also putting pressure on the system.
“We now have the globalization of drugs,” McBride said. “The demand from around the world has increased, limiting the supply of these products. It’s really scary.”
In addition to patient care, drug shortages have also derailed clinical trials meant to test the effectiveness of various chemotherapy regimens, several clinicians complained. “These shortages are also linked to ongoing or planned clinical studies because the drugs are not there for the testing,” McBride said. “So you wind up with haphazard studies and that’s very frustrating,” he said.
Also contributing to the problem is the traditional policy of manufacturers and healthcare centers to adopt “just-in-time” inventory management, intended to reduce the cost of inventory and optimize cash flow, the ASHP said in its 2009 report. While generally recognized as sound business policy for all stakeholders, during times of scarcity “this practice results in little or no inventory cushion to address short-term shortages,” the ASHP said. In a related consequence, some medical centers have begun stockpiling drug products when they get them, a strategy that “can exacerbate the shortage and divert unneeded supplies away from other health systems with patients in need,” according to the ASHP.
Another factor contributing to the shortages, according to the summit report and other industry leaders, is that manufacturers are not required by law to alert the FDA when a manufacturer discontinues a product or when the production process is interrupted. This often leaves medical centers off-guard, bewildered, and frustrated, and they frantically search for new sources of the drug. The FDA cannot force a manufacturer to produce a product, and there is no penalty for a company that does not notify the FDA when production of a drug is discontinued.
An FDA survey found that 85% of respondents had no information about why a shortage of a particular drug was occurring and no advanced warning when they discovered the shortage. This has placed an anxiety-filled burden on treatment centers to spend time searching for possible supplies of the drug from nonroutine sources or trying to find suitable alternative agents.
The lack of requirement for notification of truncated drug supply is receiving attention from Congress. US Senator Amy Klobuchar (D-Minnesota) has written to the FDA, urging the agency to step up its monitoring of the problem of shortages. “Physicians, pharmacists, and patients are currently among the last to know when an essential drug will no longer be available,” said Klobuchar. “That’s not right. There needs to be better coordination between the pharmaceutical industry, the FDA, and healthcare providers so patients don’t lose access to the medications they depend on,” the senator said.
Klobuchar said she plans to introduce legislation that would give the FDA the authority to require early notification from pharmaceutical companies when they decide to limit or discontinue production of prescription drugs.
The summit report recommends that the FDA be given the statutory authority to require manufacturers to notify the FDA when there is a single production source or when there is an interruption in the supply of raw materials, in the manufacturing process, or in active pharmaceutical ingredients.
Fox endorsed that kind of legislation, saying that one of the best things that can happen is increased transparency of the entire supply process.
Some medical centers have successfully scrambled to prevent the shortage of sterile injectables from affecting patient care. “We have definitely felt the effects [of the shortage], but so far we’ve been able to manage by borrowing and trading,” said Timothy Cox, MD, an oncologist affiliated with the St. Joseph Mercy Port Huron Health System in Michigan. That has meant that medical personnel are spending a lot of valuable time trying to find enough product, according to Cox.
He also said that the shortage has presented real problems for oncologists who prescribe combination chemotherapy regimens that require agents that are now in short supply. An example, Cox said, is the regimen he uses for patients with testicular cancer, which is cycles of BEP (bleomycin, etoposide, and cisplatin). All three of those drugs are in short supply. “And there are not a lot of options for alternatives,” he said.
Some manufacturers of sterile injectables have been able to sustain supply in their chemotherapy product lines and avoid shortages. For example, Amgen has not experienced any interruption in production, according to Mary Klem, a spokeswoman for the company’s Research and Development/ Manufacturing Division. Amgen makes a number of oncology injectables, including denosumab, filgrastim, and panitumumab.
“Our long-standing history in biotechnology manufacturing has enabled us to apply the know-how to meet the demand for oncology drugs,” Klem said. While acknowledging that shortages of product can happen to any company for a variety of reasons, Klem states that Amgen has multiple strategies in place to ensure uninterrupted production schedules.
But one of the largest manufacturers of generic sterile injectables, Teva, has confirmed problems that have led to disrupted production. Denise Bradley, senior director of corporate communications at Teva, said in a statement: “Our facility in Irvine, California, which makes our injectable products, has been on a voluntary production hold since April 2010. We anticipate beginning production at this facility again in March. We will be bringing up one manufacturing line at a time—with the focus being on those oncology products that are on the drug shortage list.”
However, she said the company would not identify the drugs for which production will be resumed. “We are not releasing the names of the drugs we’re starting production on at this time,” she said. Teva manufactures many of the most commonly used sterile injectable chemotherapy agents, including bleomycin, cisplatin, desmopressin, doxorubicin, etoposide, and leucovorin.
The welcome news that Teva was stepping up production is in line with the FDA’s forecast that manufacturers are close to reviving production output. In fact, the FDA’s Captain Jensen said, “Many of the shortages of chemotherapy drugs that occurred in 2010 are being resolved by the manufacturers; however, the leucovorin and cytarabine shortages continue to be severe. We are continuing to work on all of the shortages that have occurred and are continuing to offer assistance to all of the manufacturers on anything that will help meet patient needs.”
When asked if the FDA was confident that manufacturers can meet current demands, Jensen responded by providing a status report on the production expectations for the chemotherapy drugs. “For cisplatin, the two firms have increased supplies and report they are currently able to meet all demand. Doxorubicin is now being released by all three manufacturers, and they are all working to meet all demand. The shortage is anticipated to continue to improve based on what the manufacturers are reporting,” she said.
Furthermore, Jensen said, “Bleomycin is now being released by one firm (Teva), and two of the other manufacturers plan on releases in March—so again, based on manufacturers’ plans, this shortage is anticipated to continue to improve as well. Leucovorin has been in shortage due to one firm having manufacturing issues and the remaining firm has not been able to meet demand. A new generic firm has recently launched its leucovorin injection and it is working to increase supplies. Cytarabine has been in shortage due to delays and manufacturing problems at the three manufacturers. One of the firms is planning additional supplies to be released soon.”
Pharmaceutical companies’ plans to recharge production efforts “give us hope that they will be doing the right things for the long term,” Fox said.
What steps has the FDA taken to help manufacturers refill the supply chain of these drugs? “When there is a quality problem causing the shortage, the FDA works with the affected firm to address the issue, Captain Jensen said. “We’re also working with the remaining firms on anything that they can do to increase their production. Sometimes they will need an additional manufacturing line or an additional site or supplier qualified to help increase capacity, and (we) are glad to help with these issues to address a shortage. For leucovorin, cytarabine, and the others, FDA continues to offer assistance to all of the manufacturers and is exploring all possible options to help with supplies.”
Regarding FDA’s oversight of this problem, the summit panel recommended that the agency be given the authority to require manufacturers to provide notification 9 to 12 months before a drug is pulled off the market and to require them to have more than one production site for a sole, essential drug. “This would give the FDA the chance to help mitigate or avert the kinds of shortages we’re having now,” Benjamin said.










