
Napabucasin, a novel therapy that targets cancer stem cell pathways, is being investigated in a phase III clinical trial that is believed to be the largest study ever conducted in pancreatic ductal adenocarcinoma in the metastatic setting

Your AI-Trained Oncology Knowledge Connection!


Napabucasin, a novel therapy that targets cancer stem cell pathways, is being investigated in a phase III clinical trial that is believed to be the largest study ever conducted in pancreatic ductal adenocarcinoma in the metastatic setting

A 2018 Giants of Cancer Care® award winner for Supportive, Palliative Geriatric Care, Patricia A. Ganz, MD has spent much of her life working to improve the quality of care for patients with cancer.

The term statistically significant is almost certainly beautiful music to the ears of clinical investigators and pharma/biotech companies. However, concern develops when one inquires how the most common test of significance, the P value, is used in clinical investigative efforts and whether at times this is more harmful than helpful within the domain of cancer medicine.

Immune-based approaches have revolutionized the treatment of acute lymphoblastic lymphoma, especially for patients unable to tolerate multiagent chemotherapy. Now, investigators are looking forward to seeing these agents move into frontline settings, perhaps leading to chemotherapy-free approaches.
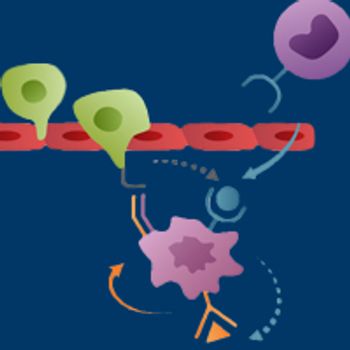
Chemokine receptor type 2, a major recruiter of circulating monocytes that subsequently develop into a protumoral type of macrophage within the tumor microenvironment, has emerged as a promising therapeutic target.

The National Cancer Institute (NCI) has designated NYU Langone Health’s Laura and Isaac Perlmutter Cancer Center as a Comprehensive Cancer Center, the highest rank awarded by the NCI. Perlmutter joins an elite group of 50 cancer centers across the country that have earned this distinction.

Although the therapeutic utility of lymphadenectomy is uncertain, it continues to provide information for staging and helps to guide postoperative adjuvant treatment for patients with endometrial cancer.

New therapeutic approaches for treating patients with earlier-stage melanoma who face a higher risk of recurrence are among the features of recently updated consensus guidelines from the Society for Immunotherapy of Cancer.

When the FDA approved its first biosimilar drug in 2015—filgrastim-sndz, for compromised white blood cell count—there was talk of the benefits of competition in the pharma industry; increased access to medications for patients; and, of course, the all-around savings. Four years later, those benefits haven’t been fully realized,

Although these therapies were initially conceived of and developed as inpatient therapies, interest is growing in extending chimeric antigen receptor T-cell therapies to the outpatient setting.