Article
Contemporary Evidence-Based Management of Newly Diagnosed Metastatic Colorectal Cancer
Author(s):
Contemporary efforts toward defining molecular subsets of colorectal cancer can potentially aid our ability to refine therapy and improve outcomes for patients with colorectal cancer.
Kanwal Raghav, MD
Abstract
The existing therapeutic armamentarium for first-line treatment of metastatic colorectal cancer is diverse and includes both cytotoxics (5-fluorouracil, capecitabine, oxaliplatin, and irinotecan) and targeted therapies (anti-vascular endothelial growth factor-A antibody [bevacizumab], and anti-epidermal growth factor receptor antibodies [cetuximab, and panitumumab]). Patients with good performance status should be treated with a combination of cytotoxics and targeted agents. Cytotoxic chemotherapy should be a doublet composed of either 5-fluorouracil or capecitabine and oxaliplatin or irinotecan (FOLFOX, CAPOX, or FOLFIRI).
To this we add one of the biologic agents, either bevacizumab for RAS mutant (mutation in KRAS and NRAS [exon 2, 3 and 4]) or bevacizumab/cetuximab/panitumumab for RAS wild-type tumors. No one combination is superior to the other and choice should be chosen based on toxicity and mutational profile. In elderly patients, limited data have demonstrated a similar benefit from multi-agent chemotherapy, albeit at the cost of increased toxicity. Combination therapy should be considered carefully in elderly patients after appropriate clinical triage using comorbidities, functional status, and comprehensive geriatric assessment tools. Treatment plan should be driven by symptom control and quality of life measures.
Management of patients presenting with potentially resectable disease requires early identification of these cases and an integrated multidisciplinary approach. Contemporary efforts toward defining molecular subsets of colorectal cancer can potentially aid our ability to refine therapy and improve outcomes for patients with colorectal cancer.
Introduction
With an estimated 1.3 million new cancer cases and 700,000 deaths worldwide, colorectal cancer (CRC) is a major cause of cancer-related morbidity and mortality globally.1 The incidence of CRC is higher in developed countries compared with developing countries, with the overall age-adjusted rate of 36.3 per 100,000 population.1 Despite the decreasing incidence of CRC, a total of 132,700 new cancer cases and 49,700 deaths are estimated to occur in the United States in 2015, making it the second most common cause of cancer-related mortality.2 About 20% of all CRC cases are metastatic at diagnosis, and approximately 30% of all stage II and III CRC cases will develop recurrent metastatic disease after initial treatment.3,4 Medical oncologists frequently encounter patients with metastatic CRC, and understanding the nuances of treating this disease is vital for improved outcomes in these patients. This review summarizes the existing evidence underlying the paradigm for treatment of metastatic CRC in the first-line setting.
Figure. Schema for Contemporary Therapy of Unresectable Metastatic Colorectal Cancer
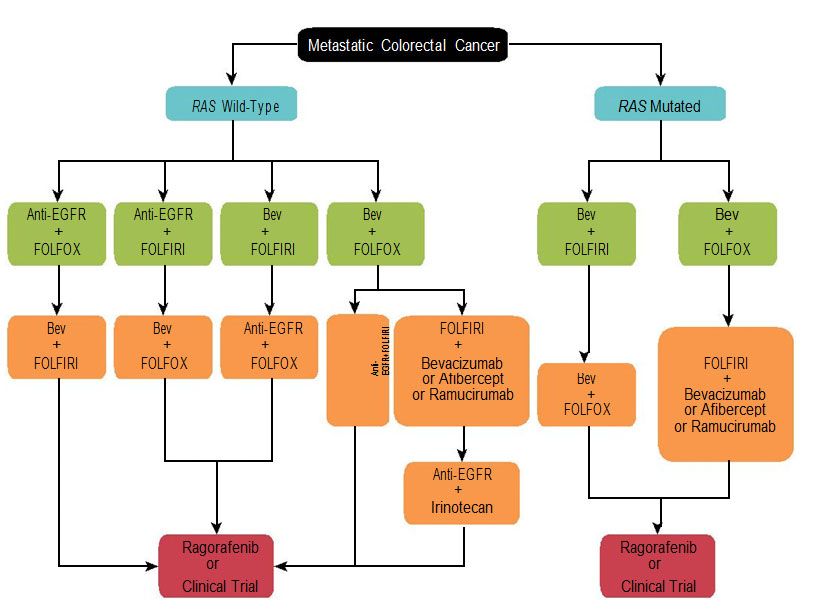
Anti-EGFR represents either cetuximab or panitumumab. 5-fluorouracil (5-FU) can be subsituted with capecitabine. Bevacizumab indicates bevacizumab; FOLFIRI, 5-FU and irinotecan; FOLFIRI, 5-FU and oxaliplatin.
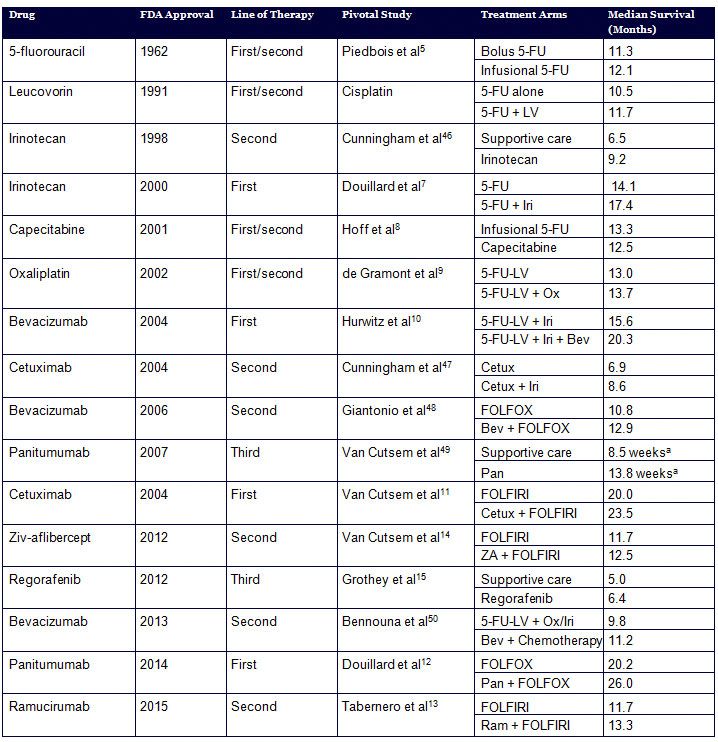
An armamentarium of therapies has accumulated over the past few decades in metastatic CRC, leading to an incremental increase in the survival of patients with this disease. However, the composite array of cytotoxic and targeted therapies has made treatment of metastatic CRC relatively complicated. Currently approved agents for treatment of metastatic CRC include cytotoxics: 5-fluorouracil (5-FU), capecitabine, oxaliplatin, and irinotecan; and targeted therapies: bevacizumab, cetuximab, panitumumab, regorafenib, ziv-aflibercept, and ramucirumab (Figure and Table).5-15 Apart from regorafenib, which is approved for the refractory setting, and aflibercept and ramucirumab, which are approved for oxaliplatin-resistant patients, all other drugs can be used in the first-line setting.13-15 Consequently, selecting the appropriate first-line therapy in patients with metastatic CRC is a complex and critical decision that medical oncologists need to make on a routine basis.
Choice of Chemotherapy Backbone
For the purpose of this review we have divided this decision into 2 main modules: the choice of chemotherapy backbone and the choice of biologic agent. The last section deals with management of metastatic CRC in elderly patients, due to the singular treatment issues in this population. The scope of this review is restricted to systemic management of metastatic CRC and does not include surgical or other locoregional management strategies. With appropriate patient selection and multidisciplinary care involving liver resection and systemic perioperative chemotherapy, a small subset of patients with limited metastatic liver disease can achieve long-term disease control and even cure.16 The 5-year survival in these select patients approaches 50%.16The fluoropyrimidine, 5-FU, has been shown to have significant activity in metastatic CRC, with response rates ranging from 15% to 30% and overall survival (OS) ranging from 57 weeks to 62 weeks.17 Modulation of 5-FU by addition of leucovorin (LV) and by administering continuous infusion has been shown to increase tumor response rates (21% for 5-FU-LV vs 11% for 5-FU alone and 22% for infusional 5-FU vs 14% for bolus 5-FU, respectively) compared with bolus 5-FU alone.5,18 Additionally, infusional regimens are associated with longer median progression-free survival (PFS; 27.6 vs 22 weeks) and lower grade 3-4 toxicities (11.1% vs 23.9%), namely granulocytopenia, diarrhea, and mucositis.17
Systemic 5-FU-based doublet chemotherapy in combination with either irinotecan (FOLFIRI) or oxaliplatin (FOLFOX) has been the standard of care for metastatic CRC for many years, and has been shown in multiple trials to improve response rate (RR), PFS, and OS compared with 5-FU alone.7,9 Randomized comparisons of FOLFOX and FOLFIRI regimens have shown similar efficacy with different toxicity profiles, namely neutropenia and neuropathy with oxaliplatin, and mucositis and diarrhea with irinotecan.19,20 The choice of chemotherapy backbone between FOLFOX and FOLFIRI is therefore dictated by toxicity; grade 3-4 mucositis, nausea/vomiting, and diarrhea are more frequent with FOLFIRI, and grade 3-4 neutropenia and neurotoxicity are more frequent with FOLFOX.19 Furthermore, in the era when resection of liver metastases has been adopted in routine management of metastatic CRC, the differential hepatotoxic effects of these agents should also be deliberated if a patient is considered to be a surgical candidate.21,22 In a retrospective analysis, oxaliplatin was associated with sinusoidal dilation, and irinotecan was associated with steatohepatitis.22 Patients with steatohepatitis had increased 90-day mortality.22 The choice and duration of chemotherapy in patients who may be surgical candidates should be discussed with surgeons as a part of a multidisciplinary approach.
Multiple trials have also established the equivalence of capecitabine and 5-FU in combination with oxaliplatin.23 Despite a meta-analysis that showed similar efficacy and tolerability of capecitabine and 5-FU in combination with irinotecan, toxicity of some concern has been reported in some trials with the use of capecitabine and irinotecan, specifically vomiting, diarrhea, and dehydration, compared with FOLFIRI.24,25 Summary: The cytotoxic chemotherapy line systemic therapy for unresectable metastatic CRC should include an infusional regimen of 5-FU or either oxaliplatin or irinotecan.
Two of 3 studies have shown a benefit to using a 3-cytotoxic-drug upfront strategy compared with doublet regimen (FOLFIRI). In a phase III study comparing fluorouracil, leucovorin, oxaliplatin, and irinotecan (FOLFOXIRI) with FOLFIRI in highly selected patients (age <75 years and ECOG PS 0 or 1) with metastaticCRC, FOLFOXIRI improved RR, PFS, and OS, with increased toxicity in patients with metastatic CRC.26
Table. Evolution of Therapeutic Interventions in Metastatic Colorectal Cancer
A similar study with less stringent inclusion criteria performed by the Hellenic Oncology Research Group failed to demonstrate superiority of FOLFOXIRI combination compared with FOLFIRI.27 The third study compared bevacizumab plus FOLFOXIRI with bevacizumab plus FOLFIRI and showed improved PFS and RR and a statistically insignificant improvement in OS (31.0 months vs 25.8 months), again at the cost of increased toxicity.28 The lack of molecular stratification and inability to adjust for second-line therapies limits the clinical applicability of this study. Therefore, although the use of 3 cytotoxics is a viable option in select patients with metastatic CRC with good performance status (PS), this benefit should be carefully weighed against the increased risk of neuropathy, diarrhea, and neutropenia.
Choice of Biologic Agent
Summary: A 3-drug cytotoxic regimen (FOLFOXIRI) is a reasonable option in younger patients with good PS, but is associated with significant toxicity.Antiangiogenic therapy with bevacizumab has shown benefit in metastatic CRC in combination with chemotherapy.29 Bevacizumab combined with irinotecan, bolus 5-FU, and leucovorin (IFL) was shown to improve survival (median OS, 20.3 months vs 15.6 months) compared with chemotherapy alone.10 Similarly, anti-EGFR therapy with cetuximab and panitumumab in combination with chemotherapy has been shown to improve PFS, RR, and OS in metastatic CRC with RAS wild-type tumors.11,30 Initial analyses showed no benefit of anti-EGFR therapy in patients harboring KRAS mutations in exon 2.11,12 More recent prospective-retrospective analyses have shown that other activating RAS mutations (KRAS exon 3 or 4 and NRAS exon 2, 3, or 4) are also negative predictive biomarkers for anti-EGFR therapy,30 and therefore use of anti-EGFR therapy should be restricted to patients with RAS wild-type tumors. Furthermore, although BRAFV600E mutation is a negative prognostic factor in metastatic CRC, current data are insufficient to restrict use of anti-EGFR agents in this subset of CRC.30 Participation of these patients in clinical trials should be encouraged due to the poor prognosis associated with this molecular subtype.
Due to their similar efficacy, cetuximab and panitumumab can be used interchangeably with either FOLFOX or FOLFIRI regimens. Multiple trials have been performed to address the issue of the optimal biologic therapy in first-line management of metastatic CRC. The largest study, the US Intergroup 80405 trial,31 in which patients with KRAS exon 2 wild-type tumors were randomized to receive either first-line cetuximab or bevacizumab with either FOLFOX or FOLFIRI, showed similar OS for all regimens. However, 2 other trials comparing efficacy of anti-VEGF therapy with anti-EGFR therapy have shown contrasting results.32,33 The FIRE-3 trial32 compared first-line FOLFIRI combined with either bevacizumab or cetuximab, and the PEAK trial33 compared FOLFOX combined with either panitumumab or bevacizumab. Both studies showed an OS benefit without a PFS benefit with anti-EGFR therapy. However, lack of details regarding therapy administered beyond the first-line treatment limits the ideal interpretation of these studies. Although the general consensus is that the various permutations and combinations of biologic therapies and chemotherapy are equivalent in metastatic CRC, the randomized EPOC study34 in metastatic CRC with resectable colorectal liver metastasis showed a shorter PFS with addition of cetuximab to pre-operative chemotherapy. The biological basis for such an interaction is unclear, and confirmatory trials are needed to address this specific question.35
Efforts at intensifying therapy using dual antibody therapy (bevacizumab and cetuximab or panitumumab) with chemotherapy in untreated metastatic CRC were investigated in the Panitumumab Advanced Colorectal Cancer Evaluation (PACCE) and CAIRO 2 trials.36,37 Both studies showed increased toxicity and a significantly shorter PFS with the dual-antibody strategy, and therefore this approach should not be used in management of metastatic CRC.
Treatment of Elderly Patients
Summary: Targeted therapy with either bevacizumab or anti-EGFR agents (for patients with RAS wild-type tumors only) should be used as a part of first-line therapy in combination with cytotoxic therapy.A substantial proportion of patients with metastatic CRC are age 75 years or older.38 The palliative intent of chemotherapy in this setting coupled with limited life expectancy, medical comorbidities, and inadequate participation in clinical trials makes management of these patients a challenging endeavor. Although studies have shown that compared with younger patients, elderly patients with metastatic CRC derive similar benefits from first-line chemotherapy with oxaliplatin-fluoropyrimidine combinations without much increase in toxicity, elderly patients are less likely to receive chemotherapy, and more specifically, combination chemotherapy.39,40 Tools beyond PS that assess a more complete health status, such as the Comprehensive Geriatric Assessment (CGA), can be used to predict treatment-related toxicity in elderly patients.41
A pooled analysis of oxaliplatin-fluorouracil chemotherapy in elderly patients from 4 clinical trials showed that the relative benefit of FOLFOX did not differ by age.42 The MRC FOCUS 2 trial,43 a randomized trial of capecitabine or 5-FU with or without dose-reduced oxaliplatin in patients considered unfit for full-dose chemotherapy, showed a trend toward longer PFS and OS with FOLFOX. The subgroup analysis of patients older than 65 years in a study comparing FOLFOXIRI with FOLFIRI as first-line treatment revealed no substantial benefit of the triple combination in terms of OS, and resulted in higher incidence of grade 3/4 adverse events compared with younger patients.44 Dosage reductions and treatment delays were also more frequent in the FOLFOXIRI arm. Use of bevacizumab in elderly patients is supported by the AVEX trial,45 a randomized study of capecitabine with or without bevacizumab, which showed improved PFS and a trend toward improved OS, but with an increased risk of thromboembolic events. Limited data exist regarding use of anti-EGFR therapy in elderly patients with metastatic CRC, but indicate similar efficacy compared with younger patients.
Although age alone should not be an exclusion criterion for the use of multi-agent chemotherapy, in light of the noninferiority of single-agent therapy in elderly patients and modest benefit with combination regimens, multi-agent treatment should be used with careful consideration for quality of life, toxicity profile, and patient preferences.
Summary: Combination chemotherapy should be used only in fit elderly patients with good PS. In frail elderly patients, sequential single-agent therapy should be used with caution.
Affiliations: Kanwal Raghav, MD, and Cathy Eng, MD, are from the Department of Gastrointestinal Medical Oncology, The University of Texas MD Anderson Cancer Center, Houston.
Disclosures: Drs Raghav and Eng report no relevant financial conflicts of interest to disclose. Address correspondence to: Kanwal Raghav, MD, Assistant Professor, Department of Gastrointestinal Medical Oncology, The University of Texas MD Anderson Cancer Center, 1515 Holcombe Blvd, Unit 426, Houston, TX 77030. Phone: 713-792-2828; email: kpraghav@mdanderson.org.
References
- Torre LA, Bray F, Siegel RL, et al. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65:87-108.
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA Cancer J Clin. 2015;65:5-29.
- Chen VW, Hsieh MC, Charlton ME, et al. Analysis of stage and clinical/prognostic factors for colon and rectal cancer from SEER registries: AJCC and collaborative stage data collection system. Cancer. 2014;120(suppl 23):3793-3806.
- Andre T, Boni C, Navarro M et al. Improved overall survival with oxaliplatin, fluorouracil, and leucovorin as adjuvant treatment in stage II or III colon cancer in the MOSAIC trial. J Clin Oncol. 2009;27:3109-3116.
- Kohne CH, Wils J, Lorenz M, et al. Randomized phase III study of high-dose fluorouracil given as a weekly 24-hour infusion with or without leucovorin versus bolus fluorouracil plus leucovorin in advanced colorectal cancer: European Organisation for Research and Treatment of Cancer Gastrointestinal Group Study 40952. J Clin Oncol. 2003;21:3721-3728.
- Douillard JY, Cunningham D, Roth AD, et al. Irinotecan combined with fluorouracil compared with fluorouracil alone as first-line treatment for metastatic colorectal cancer: a multicentre randomised trial. Lancet. 2000;355:1041-1047.
- Hoff PM, Ansari R, Batist G, et al. Comparison of oral capecitabine versus intravenous fluorouracil plus leucovorin as first-line treatment in 605 patients with metastatic colorectal cancer: results of a randomized phase III study. J Clin Oncol. 2001;19:2282-2292.
- Hurwitz H, Fehrenbacher L, Novotny W, et al. Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N Engl J Med. 2004;350:2335-2342.
- Van Cutsem E, Kohne CH, Lang I, et al. Cetuximab plus irinotecan, fluorouracil, and leucovorin as first-line treatment for metastatic colorectal cancer: updated analysis of overall survival according to tumor KRAS and BRAF mutation status. J Clin Oncol. 2011;29:2011-2019.
- Douillard JY, Siena S, Cassidy J, et al. Final results from PRIME: randomized phase III study of panitumumab with FOLFOX4 for first-line treatment of metastatic colorectal cancer. Ann Oncol. 2014;25:1346-1355.
- Tabernero J, Yoshino T, Cohn AL, et al. Ramucirumab versus placebo in combination with second-line FOLFIRI in patients with metastatic colorectal carcinoma that progressed during or after first-line therapy with bevacizumab, oxaliplatin, and a fluoropyrimidine (RAISE): a randomised, double-blind, multicentre, phase 3 study. Lancet Oncol. 2015;16:499-508.
- Van Cutsem E, Tabernero J, Lakomy R, et al. Addition of aflibercept to fluorouracil, leucovorin, and irinotecan improves survival in a phase III randomized trial in patients with metastatic colorectal cancer previously treated with an oxaliplatin-based regimen. J Clin Oncol. 2012;30:3499-3506.
- Grothey A, Van Cutsem E, Sobrero A, et al. Regorafenib monotherapy for previously treated metastatic colorectal cancer (CORRECT): an international, multicentre, randomised, placebo-controlled, phase 3 trial. Lancet. 2013;381:303-312.
- Nordlinger B, Sorbye H, Glimelius B, et al. Perioperative FOLFOX4 chemotherapy and surgery versus surgery alone for resectable liver metastases from colorectal cancer (EORTC 40983): long-term results of a randomised, controlled, phase 3 trial. Lancet Oncol. 2013;14:1208-1215.
- Thirion P, Michiels S, Pignon JP, et al. Modulation of fluorouracil by leucovorin in patients with advanced colorectal cancer: an updated meta-analysis. J Clin Oncol. 2004;22:3766-3775.
- Tournigand C, Andre T, Achille E, et al. FOLFIRI followed by FOLFOX6 or the reverse sequence in advanced colorectal cancer: a randomized GERCOR study. J Clin Oncol. 2004;22:229-237.
- Colucci G, Gebbia V, Paoletti G, et al. Phase III randomized trial of FOLFIRI versus FOLFOX4 in the treatment of advanced colorectal cancer: a multicenter study of the Gruppo Oncologico Dell’Italia Meridionale. J Clin Oncol. 2005;23:4866-4875.
- Brouquet A, Vauthey JN, Contreras CM, et al. Improved survival after resection of liver and lung colorectal metastases compared with liver-only metastases: a study of 112 patients with limited lung metastatic disease. J Am Coll Surg. 2011;213:62-69; discussion 69-71.
- Vauthey JN, Pawlik TM, Ribero D, et al. Chemotherapy regimen predicts steatohepatitis and an increase in 90-day mortality after surgery for hepatic colorectal metastases. J Clin Oncol. 2006;24:2065-2072.
- Diaz-Rubio E, Tabernero J, Gomez-Espana A, et al. Phase III study of capecitabine plus oxaliplatin compared with continuous infusion fluorouracil plus oxaliplatin as first-line therapy in metastatic colorectal cancer: final report of the Spanish Cooperative Group for the Treatment of Digestive Tumors Trial. J Clin Oncol. 2007;25:4224-4230.
- Guo Y, Shi M, Shen X, et al. Capecitabine plus irinotecan versus 5-FU/leucovorin plus irinotecan in the treatment of colorectal cancer: a meta-analysis. Clin Colorectal Cancer. 2014;13:110-118.
- Fuchs CS, Marshall J, Mitchell E, et al. Randomized, controlled trial of irinotecan plus infusional, bolus, or oral fluoropyrimidines in first-line treatment of metastatic colorectal cancer: results from the BICC-C Study. J Clin Oncol. 2007;25:4779-4786.
- Falcone A, Ricci S, Brunetti I, et al. Phase III trial of infusional fluorouracil, leucovorin, oxaliplatin, and irinotecan (FOLFOXIRI) compared with infusional fluorouracil, leucovorin, and irinotecan (FOLFIRI) as first-line treatment for metastatic colorectal cancer: the Gruppo Oncologico Nord Ovest. J Clin Oncol. 2007;25:1670-1676.
- Souglakos J, Androulakis N, Syrigos K, et al. FOLFOXIRI (folinic acid, 5-fluorouracil, oxaliplatin and irinotecan) vs FOLFIRI (folinic acid, 5-fluorouracil and irinotecan) as first-line treatment in metastatic colorectal cancer (MCC): a multicentre randomised phase III trial from the Hellenic Oncology Research Group (HORG). Br J Cancer. 2006;94:798-805.
- Loupakis F, Cremolini C, Masi G, et al. Initial therapy with FOLFOXIRI and bevacizumab for metastatic colorectal cancer. N Engl J Med. 2014;371:1609-1618.
- Hurwitz HI, Tebbutt NC, Kabbinavar F, et al. Efficacy and safety of bevacizumab in metastatic colorectal cancer: pooled analysis from seven randomized controlled trials. Oncologist. 2013;18:1004-1012.
- Douillard JY, Oliner KS, Siena S, et al. Panitumumab- FOLFOX4 treatment and RAS mutations in colorectal cancer. N Engl J Med. 2013;369:1023-1034.
- Venook AP, Niedzwiecki D, Lenz H-J, et al. CALGB/SWOG 80405: phase III trial of irinotecan/5-FU/leucovorin (FOLFIRI) or oxaliplatin/5-FU/leucovorin (mFOLFOX6) with bevacizumab (BV) or cetuximab (CET) for patients (pts) with KRAS wild-type (wt) untreated metastatic adenocarcinoma of the colon or rectum (MCRC) (abstract). J Clin Oncol. 2014;32(suppl 5s; abstr LBA3).
- Heinemann V, von Weikersthal LF, Decker T, et al. FOLFIRI plus cetuximab versus FOLFIRI plus bevacizumab as first-line treatment for patients with metastatic colorectal cancer (FIRE-3): a randomised, open-label, phase 3 trial. Lancet Oncol. 2014;15:1065-1075.
- Schwartzberg LS, Rivera F, Karthaus M, et al. PEAK: a randomized, multicenter phase II study of panitumumab plus modified fluorouracil, leucovorin, and oxaliplatin (mFOLFOX6) or bevacizumab plus mFOLFOX6 in patients with previously untreated, unresectable, wild-type KRAS exon 2 metastatic colorectal cancer. J Clin Oncol. 2014;32:2240-2247.
- Nordlinger B, Poston GJ, Goldberg RM. Should the results of the new EPOC trial change practice in the management of patients with resectable metastatic colorectal cancer confined to the liver? J Clin Oncol. 2015;33:241-243.
- Hecht JR, Mitchell E, Chidiac T, et al. A randomized phase IIIB trial of chemotherapy, bevacizumab, and panitumumab compared with chemotherapy and bevacizumab alone for metastatic colorectal cancer. J Clin Oncol. 2009;27(5):672-680.
- Tol J, Koopman M, Cats A, et al. Chemotherapy, bevacizumab, and cetuximab in metastatic colorectal cancer. N Engl J Med. 2009;360(6):563-572.
- Sastre J, Aranda E, Massuti B, et al. Elderly patients with advanced colorectal cancer derive similar benefit without excessive toxicity after first-line chemotherapy with oxaliplatin-based combinations: comparative outcomes from the 03-TTD-01 phase III study. Crit Rev Oncol Hematol. 2009;70:134-144.
- Ho C, Ng K, O’Reilly S, Gill S. Outcomes in elderly patients with advanced colorectal cancer treated with capecitabine: a population-based analysis. Clin Colorectal Cancer. 2005;5:279-282.
- Kenis C, Decoster L, Van Puyvelde K, et al. Performance of two geriatric screening tools in older patients with cancer. J Clin Oncol. 2014;32:19-26.
- Goldberg RM, Tabah-Fisch I, Bleiberg H, et al. Pooled analysis of safety and efficacy of oxaliplatin plus fluorouracil/ leucovorin administered bimonthly in elderly patients with colorectal cancer. J Clin Oncol. 2006;24:4085-4091.
- Seymour MT, Thompson LC, Wasan HS, et al. Chemotherapy options in elderly and frail patients with metastatic colorectal cancer (MRC FOCUS2): an open-label, randomised factorial trial. Lancet. 2011;377:1749-1759.
- Vamvakas L, Athanasiadis A, Karampeazis A, et al. Clinical outcome of elderly patients with metastatic colorectal cancer treated with FOLFOXIRI versus FOLFIRI: subgroup analysis of a randomized phase III trial from the Hellenic Oncology Research Group (HORG). Crit Rev Oncol Hematol. 2010;76:61-70.
- Cunningham D, Lang I, Marcuello E, et al. Bevacizumab plus capecitabine versus capecitabine alone in elderly patients with previously untreated metastatic colorectal cancer (AVEX): an open-label, randomised phase 3 trial. Lancet Oncol. 2013;14:1077-1085.
- Cunningham D, Pyrhönen S, James RD, et al. Randomised trial of irinotecan plus supportive care versus supportive care alone after fluorouracil failure for patients with metastatic colorectal cancer. Lancet. 1998;352(9138):1413-1418.
- Cunningham D, Humblet Y, Siena S, et al. Cetuximab monotherapy and cetuximab plus irinotecan in irinotecan-refractory metastatic colorectal cancer. N Engl J Med. 2004;351(4):337-345.
- Giantonio BJ, Catalano PJ, Meropol NJ, et al. Bevacizumab in combination with oxaliplatin, fluorouracil, and leucovorin (FOLFOX4) for previously treated metastatic colorectal cancer: results from the Eastern Cooperative Oncology Group Study E3200. J Clin Oncol. 2007;25(12):1539-1544.
- Van Cutsem E, Peeters M, Siena S, et al. Open-label phase III trial of panitumumab plus best supportive care compared with best supportive care alone in patients with chemotherapy-refractory metastatic colorectal cancer. J Clin Oncol. 2007;25(13):1658-1664.
- Bennouna J, Sastre J, Arnold D, et al. Continuation of bevacizumab after first progression in metastatic colorectal cancer (ML18147): a randomised phase 3 trial. Lancet Oncol. 2013;14(1):29-37.
Reprinted with permission from American Journal of Hematology / Oncology®









