
Phase Ib/II research has shown promise for adoptive cellular therapy combined with ipilimumab in patients with advanced sarcomas.

Your AI-Trained Oncology Knowledge Connection!


Phase Ib/II research has shown promise for adoptive cellular therapy combined with ipilimumab in patients with advanced sarcomas.

Combining the investigational antibody TRC105 with Votrient showed promising antitumor activity in patients with soft tissue sarcoma.

Researchers from United States and Ireland have formed a partnership to advance nanoparticle-based delivery systems that would not only drive sustained-release of cytotoxic agents, but also could improve penetration of these treatments within the tumor and allow them to continue working for days or weeks.

Although treatments and cure rates have increased significantly over the past 60 years for patients with Hodgkin lymphoma, it is crucial that practitioners stay up-to-date on research that can affect outcomes for their patients with this uncommon form of cancer.

The FDA has granted a breakthrough therapy designation to pembrolizumab (Keytruda), an anti-PD-1 therapy, for the treatment of patients with non-small cell lung cancer (NSCLC) who are EGFR mutation- or ALK rearrangement-negative and whose disease has progressed on or following platinum-based chemotherapy.

One of the first studies to prospectively examine women's breast surgery preferences has revealed that newly diagnosed women with breast cancer who decide to undergo contralateral prophylactic mastectomy (CPM), may be making this decision as result of high anxiety and fear of recurrence, rather than relying on evidence-based information.

Long-term follow-up results demonstrated nearly doubled median OS with the combination of ipilimumab and nivolumab compared with either agent alone.

A joint analysis of two phase III trials involving a total of 4690 premenopausal women with hormone-receptor–positive (HR+) breast cancer demonstrated that adjuvant use of the aromatase inhibitor (AI), exemestane, reduced relative risk of developing subsequent invasive cancer by 28% compared with tamoxifen when both agents were combined with ovarian function suppression (OFS).

MPDL3280A reduced tumors in 43% of patients with previously treated PD-L1-positive metastatic bladder cancer, resulting in the first breakthrough therapy designation from the FDA for a treatment in this setting.

For patients with less than a year to live, the discontinuation of statins may increase the median time-to-death and improve quality of life.

Increasing efficacy with immunotherapies in some cancers led to the strategy being deemed Breakthrough of the Year by Science magazine in 2013

A vast, pooled retrospective analysis has demonstrated that the use of ASIs may significantly improve survival outcomes in patients with mRCC.

Data from an interim analysis of an ongoing phase II study suggest that patients with carcinoid and pituitary NETs, who have been traditionally chemo-resistant, may obtain "extraordinary responses" with capecitabine and temozolomide.

In the United States, the incidence of lung cancer has increased in those aged >74 years and will likely continue to rise.

A conversation with Denise R. Aberle, MD, on the National Lung Screening Trial (NLST) at the 12th International Lung Cancer Congress.

Although researchers know that estrogen plays a role in most cases of breast cancer, they don't fully understand how.

Immunotherapies made headlines in oncology last month when the Centers for Medicare & Medicaid Services (CMS) decided that the anticancer vaccine sipuleucel-T (Provenge) will be a payable expense for prostate cancer.

Therapies that target bone metastases have been developed for more than a decade.

A large-scale study of HPV testing and Pap smear for cervical cancer screening confirms that women can safely extend screening intervals from 1 to 3 years.
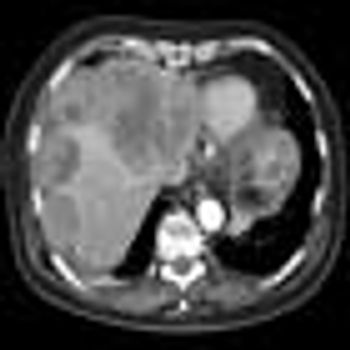
Certain patients whose cancer cells form mutations and build up a resistance to drugs, drive the need for second- and third-line therapies

Spectrum Pharmaceuticals stock prices are the biggest story across the internet, not the FDA's approval of Fusilev
Researchers have sequenced 38 tumor genomes from patients with multiple myeloma (MM), comparing them with normal DNA.

The American Society of Hematology (ASH) is sounding a fresh alarm about ongoing supply problems with oncology drugs

Cervical cancer affects about 470,000 women each year and now the disease has a new tool to combat its spread

Shirnett Williamson, MD, PhD and Derrick Grant, PhD constitute a living definition of translational oncology

Traditional, linear regimen of prostate cancer treatment may need to be reexamined as oncologists learn new ways to incorporate new therapies

Initial clinical findings indicate that results with radiotherapies sometime improve when combined with immunotherapy

Pfizer's sunitinib provides a favorable benefit-risk profile in patients with unresectable pancreatic neuroendocrine tumors (pNETs)

Immunotherapies again made headlines in oncology last month
Lung cancer in nonsmokers may soon have an adjunctive blood test for revealing common biomarkers
Published: March 14th 2011 | Updated:

Published: March 23rd 2011 | Updated:

Published: March 28th 2011 | Updated:
Published: April 6th 2011 | Updated:

Published: April 13th 2011 | Updated:

Published: April 20th 2011 | Updated: