Precision Medicine in Oncology®
Latest News

Continued Emergence of Targeted Therapies Reinforces Need for Frequent Genetic Testing in NSCLC
Latest Videos

CME Content
More News

Julian Schink, MD, discusses a study evaluating racial differences in the mutational landscape of serous endometrial cancer, underscores the need for appropriate genomic testing and treatment for Black women with the disease, and explains the importance of racial representation across clinical trials.

Although tumor mutational burden is established as a clinically informative feature of tumors, its optimal use in therapeutic decision-making faces many challenges, and we are only beginning to fully understand its strengths and limitations.
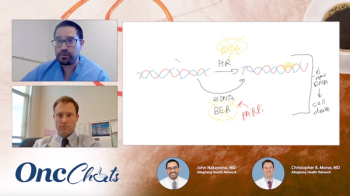
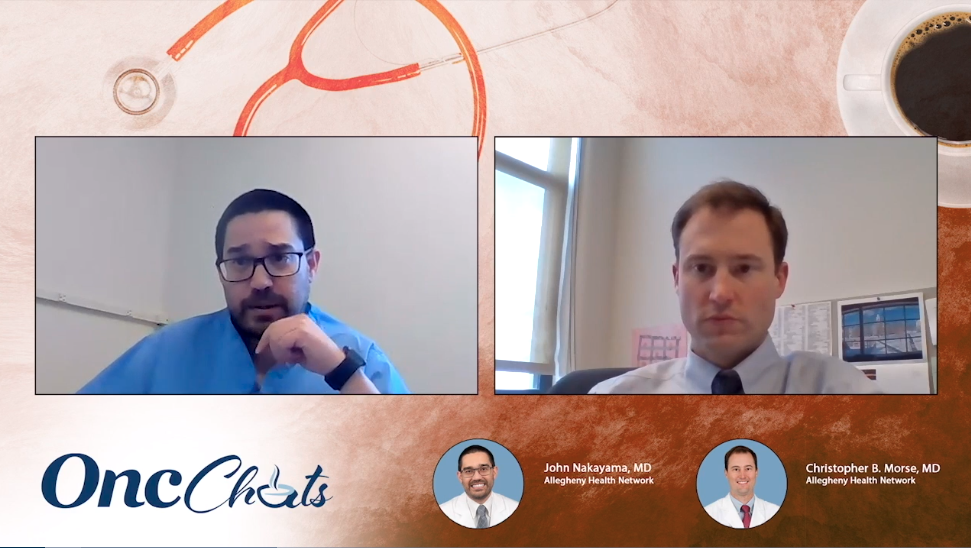
In this second episode of OncChats: Taking Action to Individualize Ovarian Cancer Care, John Nakayama, MD, and Christopher Morse, MD, discuss how to counsel patients with ovarian cancer whose tumors harbor BRCA mutations.

The Guardant Reveal assay has been expanded for the detection of minimal residual disease and disease recurrence in patients with early-stage breast cancer and lung cancer.

The FDA has approved the Oncomine Dx Target Test as a companion diagnostic to identify patients with NSCLC harboring an activating HER2 mutation who may derive clinical benefit from treatment with fam-trastuzumab deruxtecan-nxki.

Vivek Subbiah, MD, discusses the significance of the approval of dabrafenib plus trametinib for adult and pediatric patients 6 years or older with unresectable or metastatic BRAF V600E–mutant solid tumors and highlights future directions for tumor agnostic drug development.

The FDA has expanded its approval of the VENTANA MMR RxDx panel to identify patients with mismatch repair–deficient solid tumors and as a companion diagnostic assay to determine eligibility for pembrolizumab as a treatment for patients with mismatch repair–proficient endometrial cancer.
In this first episode of OncChats: Taking Action to Individualize Ovarian Cancer Care, John Nakayama, MD, and Christopher Morse, MD, discuss how BRCA mutational status affects treatment decisions for patients with ovarian cancer.

Giuseppe Lo Russo, MD, PhD, highlights the future role of liquid biopsy, the evolution of targeted therapies for KRAS G12C mutations, data to look forward to with EGFR-targeted agents, and the striking effects the COVID-19 pandemic has had on the mortality rates of patients with lung cancer.

The first-in-class p53 reactivator, PC14586, induced a response in approximately 1 of 4 patients with advanced solid tumors harboring p53 Y220C mutations and showcased an acceptable safety profile consisting primarily of grade 1 and 2 events.

During the past several decades, cancer research has produced revolutionary discoveries leading to dramatic results for many patients.

The LAG-3 pathway has emerged as the next target for the use of immune checkpoint inhibitors in oncology.

Vivek Subbiah, MD, discusses the FDA approval of dabrafenib plus trametinib for the treatment of patients with BRAF V600E–mutated unresectable or metastatic solid tumors.

Jacob J. Adashek, DO, discusses how targeting molecular alterations is key for treating multiple types of cancer.

The FDA has approved crizotinib for adult and pediatric patients aged 1 year and older with unresectable, recurrent, or refractory inflammatory ALK-positive myofibroblastic tumors.

The FDA has granted an orphan drug designation to PBI-200 for the treatment of patients with NTRK fusion–positive solid tumors, including primary and metastatic brain tumors.

The role of NTRK gene fusions across multiple cancer types have led investigators to confirm the marker in real-world data analyses.

A second-line combination regimen comprised of RGX-202-01, FOLFIRI and bevacizumab demonstrated an encouraging efficacy signal and a favorable toxicity profile in patients with KRAS-mutant colorectal cancer.

Eunice Wang, MD, discusses the long-term results of a phase 2 trial investigating crenolanib plus chemotherapy in adult patients with newly diagnosed FLT3-mutant acute myeloid leukemia.

The FDA has granted an accelerated approval to dabrafenib plus trametinib for the treatment of adult and pediatric patients aged 6 years and older with unresectable or metastatic solid tumors harboring a BRAF V600E mutation who have progressed following previous treatment and who have no satisfactory alternative treatment options.
The China National Medical Products Administration’s Center for Drug Evaluation granted 2 breakthrough therapy designations to repotrectinib for the treatment of patients with ROS1-positive metastatic non–small cell lung cancer.

Sotorasib demonstrated clinically meaningful activity and acceptable tolerability in heavily pretreated patients with KRAS G12C–mutated advanced pancreatic cancer, according to date from the single-arm, phase 1/2 CodeBreak 100 trial.

The addition of quizartinib to standard induction and consolidation chemotherapy and then continued as a single agent doubled median overall survival vs standard chemotherapy alone in patients with newly diagnosed FLT3-ITD–positive acute myeloid leukemia.
B7-H3, a member of the same protein family as PD-L1, has garnered attention as one of a slew of alternative targets riding the wave of success of immune checkpoint inhibitors in cancer immunotherapy.

The FDA has given the green light to FoundationOne CDx to identify patients with ROS1 fusion–positive non–small cell lung cancer or NTRK fusion–positive cancers who might be candidates for entrectinib.