CAR T-cell Therapy
Latest News
Latest Videos

CME Content
More News
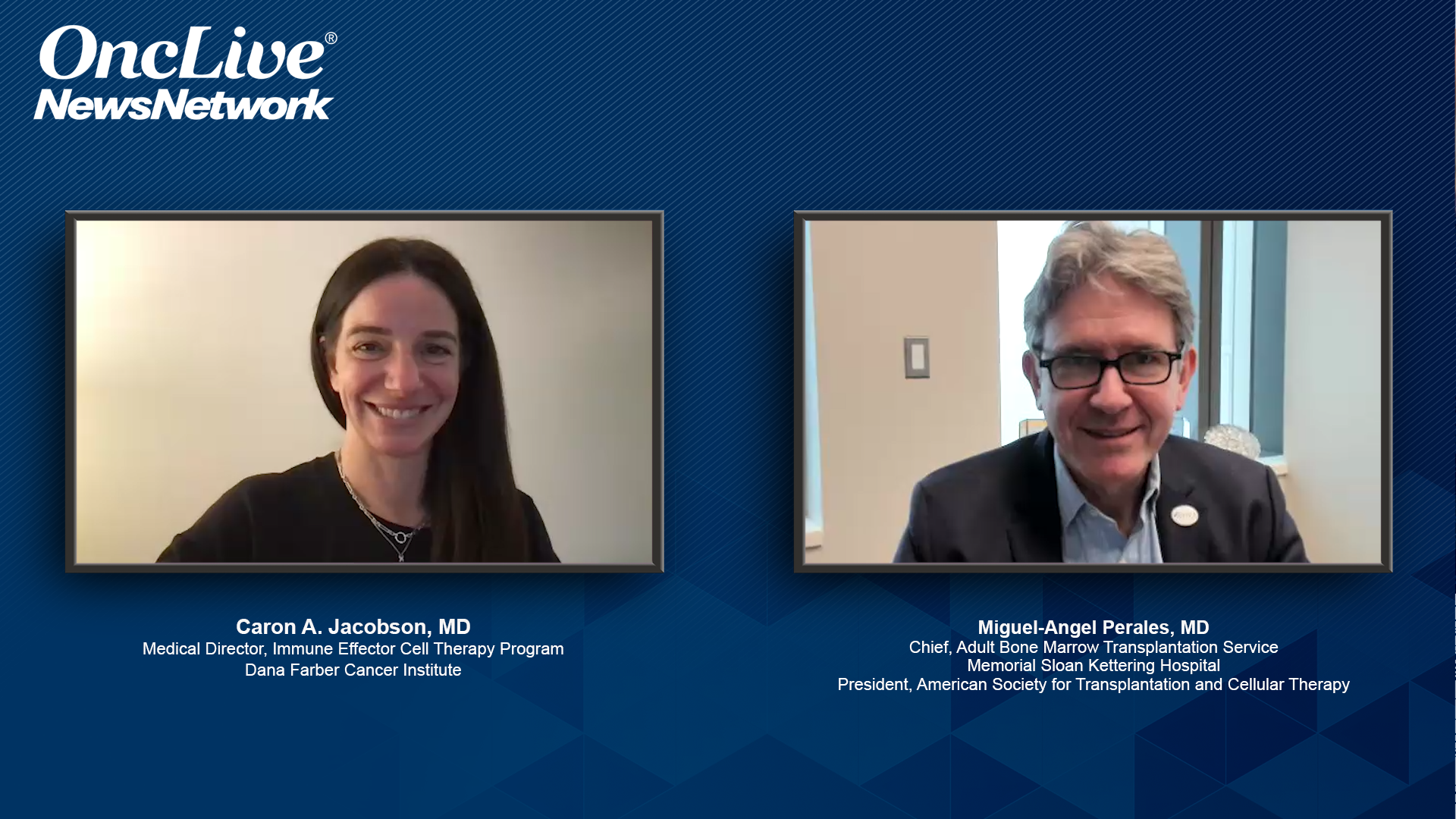
Caron A. Jacobson, MD, and Miguel-Angel Perales, MD, discuss the trajectory and role of of CAR T-cell therapy within the treatment spectrum for LBCL.

Francesco Di Meo, PhD, discusses the potential efficacy of CAR T-cells targeting the immunoreceptor LILRB4 in multiple myeloma.

The FDA has called to add a class-wide boxed warning for CAR T-cell therapies to alert patients of the risk of developing secondary T-cell malignancies.

The FDA has accepted for review the BLA for obecabtagene autoleucel's use in patients with relapsed or refractory B-cell acute lymphoblastic leukemia.

Evandro D. Bezerra, MD, discusses real-world outcomes associated with brexucabtagene autoleucel in relapsed/refractory B-cell acute lymphoblastic leukemia.

Ciara Freeman, MD, PhD, outlines an analysis conducted to identify factors linked to CAR T-cell therapy response in patients with hematologic malignancies.

Michael Jain, MD, discusses the importance of evaluating the impact of lymphoma-associated CD39-positive T cells on CAR T-cell phenotypes.
Lori A. Leslie, MD, discusses the efficacy and safety of second-line axicabtagene ciloleucel in elderly patients with high-risk, relapsed/refractory large B-cell lymphoma.

Maurie Markman, MD, discusses current challenges faced with developing robust decision support tools for oncologists and projects additional research efforts dedicated to finding a way to leverage CAR T-cell therapies in solid tumors to improve patient outcomes.

Maurie Markman, MD, highlights inroads made with immunotherapy, antibody-dependent cytotoxic agents, and molecular testing across oncology.

The FDA has approved label updates for zanubrutinib to include data from the ALPINE trial in relapsed/refractory chronic lymphocytic leukemia, and axicabtagene ciloleucel to include findings from the primary overall survival analysis of the ZUMA-7 trial in relapsed/refractory large B-cell lymphoma.

Patients with relapsed/refractory follicular lymphoma experienced durable responses when treated with tisagenlecleucel (Kymriah) in the phase 2 ELARA study (NCT03568461), according to data presented at the 2023 ASH Annual Meeting.

The administration of axicabtagene ciloleucel in the second-line setting improved overall survival and progression-free survival vs standard-of-care treatment in patients with relapsed/refractory large B-cell lymphoma who are at least 65 years of age.

The utilization of remote patient monitoring within the first 30 days of outpatient CAR T-cell therapy can promote patient safety, reduce costs, and decrease hospitalization rates by allowing patients with hematologic malignancies to connect with a virtual care platform.
Idecabtagene vicleucel resulted in meaningful improvements in symptoms, functioning, overall health status, and health-related quality of life vs standard regimens in select patients with triple-class exposed relapsed/refractory multiple myeloma, according to updated data from the phase 3 KarMMa-3 trial.

Shortages of several anticancer drugs continue to negatively impact clinicians’ ability to deliver the best possible care to patients.

Michael Hurwitz, MD, PhD, discusses the ongoing investigation into the use of CAR T-cell therapies in patients with solid tumors, such as kidney cancers.

Adnan F. Danish, MD, discusses findings from a study evaluating the prevalence of patients with high-risk non-Hodgkin lymphoma who were eligible for bridging radiation therapy prior to receiving CAR T-cell therapy, as well as the outcomes of these patients following CAR T-cell therapy.

The FDA has received reports of T-cell malignancies in patients who have been treated with CD19- or BCMA-directed autologous CAR T-cell immunotherapies.

Adrienne Phillips, MD, MPH, discusses the impact of CAR T-cell therapy on the treatment paradigm for patients with large B-cell lymphoma, highlighting key advancements in the decision-making process for CAR T-cell therapy selection.

A biologics license application seeking the approval of obecabtagene autoleucel for the treatment of adult patients with relapsed/refractory B-cell acute lymphoblastic leukemia has been submitted to the FDA.

Michael Hurwitz, MD, PhD, discusses the feasibility of utilizing CAR T-cell therapies in patients with solid tumors, such as kidney cancers.

Lori A. Leslie, MD, discusses the benefits of implementing CAR T-cell therapy programs for patients with hematologic malignancies and highlights the successes with administering CAR T-cell therapy that JTCC has experienced.

Adrienne Phillips, MD, MPH, discusses the management of toxicities associated with CAR T-cell therapy across hematologic malignancies.

Md Kamrul Hasan, PhD, discusses the preclinical efficacy and safety of ROR1-specific CAR T-cells in a preclinical mouse model of chronic lymphocytic leukemia.