Lung Cancer
Latest News
Latest Videos

CME Content
More News







Merck announced the signing of three separate clinical collaboration agreements to evaluate the potential of its investigational anti-PD-1 immunotherapy MK-3475 across multiple tumor types
Roy S. Herbst, MD, PhD, a professor of medicine at the Yale Cancer Center and chief of medical oncology at Smilow Cancer Hospital at Yale-New Haven in Connecticut, discusses the unique benefit of targeting the PD-1/PD-L1 pathway in lung cancer.

Treatment of stage III non-small cell lung cancer (NSCLC) with chemotherapy, radiation, and surgery has hit a plateau, and the key to improved outcomes will hinge on the testing of targeted therapies in clinical trials with more novel designs and better patient selection, according to a leading researcher.
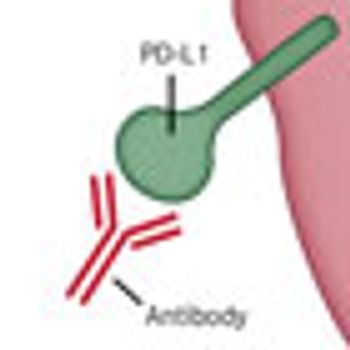
Immunotherapy has become an increasingly appealing therapeutic strategy for patients with cancer, with many late-stage clinical trials demonstrating overall survival (OS) advantages in melanoma and castrationresistant prostate cancer.

Frederick Alan Rapoport, MD, clinical instructor in the Department of Medicine at NYU Langone Medical Center, describes the mechanism and utility of Samsca (tolvaptan).

Mark G. Kris, MD, from the Memorial Sloan-Kettering Cancer Center, describes the new drugs and other emerging trends in lung cancer.
Bilal Piperdi, MD, Associate Professor of Clinical Medicine, Department of Medicine (Oncology), Albert Einstein College of Medicine, discusses the potential of immunotherapy for the treatment of patients with lung cancer.

The BRAF inhibitor dabrafenib (Tafinlar) has received a Breakthrough Therapy designation from the FDA for its potential as a treatment for patients with metastatic BRAF V600E mutation-positive non-small cell lung cancer (NSCLC) who have received at least one prior line of platinum-containing chemotherapy.

Mark G. Kris, MD, William and Joy Ruane Chair in Thoracic Oncology, Memorial Sloan Kettering Cancer Center, discusses some of the challenges of treating patients with lung cancer.

There has been considerable discussion within the oncology literature during the past several years regarding the level of evidence required to consider a new antineoplastic agent an acceptable "standard-of-care" in routine disease management.
Alice T. Shaw, MD, PhD, discusses how crizotinib has changed patient care over the last few years.

Naiyer A. Rizvi, MD, an associate attending physician, Memorial Sloan-Kettering Cancer Center, discusses the phenomenon of pseudoprogression in patients with lung cancer after they receive immunotherapy treatment.

On December 30th, the United States Preventive Services Task Force (USPSTF) announced they recommend annual screening for lung cancer with low-dose computed tomography (LDCT) in adults aged 55-80 years who have a 30 pack-year smoking history.

Between 2007 and 2011, a collaboration among clinical oncologists, pathologists, and industry scientists led to the identification of a new molecularly defined subset of non-small cell lung cancer, followed by the finding that crizotinib, then under development as a MET inhibitor, was an inhibitor of anaplastic lymphoma kinase.
Chandra P. Belani, MD, discusses CO-1686, which is currently being studied for the treatment of non-small cell lung cancer.

Roy S. Herbst, MD, PhD, discusses the sequencing possibilities of anti-PD-1 agents in treating patients with lung cancer.

Bilal Piperdi, MD, from the Albert Einstein College of Medicine, discussed a trial looking at a vaccine in patients with lung cancer at the 8th Annual New York Lung Cancer Symposium®.

Mark G. Kris, MD, William and Joy Ruane Chair in Thoracic Oncology, Memorial Sloan Kettering Cancer Center, discusses the future of lung cancer treatment at the 8th Annual New York Lung Cancer Symposium®
Drug research and development continues to focus on regimens that select therapy according to the pathologic and molecular characteristics of the tumor.