Publication
Article
Supplements and Featured Publications
Novel CDK9 Inhibitor Looks to Expand Options in MYC-Amplified Solid Tumors and Non-Hodgkin Lymphoma
Author(s):
Investigators are aiming to add another agent to the treatment armamentarium for patients with relapsed or refractory solid tumors, as well as those with non-Hodgkin lymphoma, with the development of KB-0742, a CDK9 inhibitor.
Miguel Villalona-Calero, MD

Investigators are aiming to add another agent to the treatment armamentarium for patients with relapsed or refractory solid tumors, as well as those with non-Hodgkin lymphoma (NHL), with the development of KB-0742, a CDK9 inhibitor. It is set to be evaluated in a phase 1/2 trial (NCT04718675) that has begun to recruit patients.1
KB-0742 is a selective CDK9-directed agent that inhibits the gene transcription of multiple antiapoptotic proteins and oncogenic transcription factors, such as MYC and androgen receptors. The binding of KB-0742 to CDK9 leads to cell cycle arrest and apoptosis and prevents tumor cell proliferation.2
“Key factors of importance for this compound are that it’s oral, which is welcomed by patients [as an aspect that increases] quality of life,” Miguel Villalona-Calero, MD, said in an interview with OncologyLive. “With certain drugs, sometimes [patients] require a higher dose over a shorter period to hit the target, which implies that [the drugs will also] hit things that are not the target, [demonstrating] off-target action. And then the target action is sometimes limited, [as the drug] doesn’t stay to downregulate the target for a long time. This drug, based on the preclinical data we have available, has a long half-life and therefore stays and interacts with the target for longer. It also has selectivity, so it has fewer off-target effects compared with other agents.”
Villalona-Calero is the director of the Early Phase Therapeutics Program, coprogram leader of the Developmental Cancer Therapeutics Program, and a professor in the Department of Medical Oncology & Therapeutics Research at City of Hope in Duarte, California.
Leveraging MYC
Villalona-Calero noted that MYC is active and amplified in several malignancies, including ovarian cancer, triple-negative breast cancer (TNBC), and double-hit lymphoma. CDK9 is a MYC-distal super enhancer that phosphorylates RNA polymerase II and regulates the transcription of key oncogenes, he said. In preclinical findings presented during the American Association for Cancer Research 2023 Annual Meeting, investigators evaluated KB-0742 in 11 tumor types using immortalized cell lines, patient-derived cell lines, patient-derived organoids, and patient-derived xenografts. Head and neck, esophageal, gastric, liver, pancreatic, ovarian, prostate, and colon cancers and TNBC, non–small cell lung cancer (NSCLC), and lymphoma were all examined.3
Results from preclinical testing designed to further evaluate the correlation between MYC and sensitivity to treatment with KB-0742 showed that minimal activity was observed with the agent in head and neck, esophageal, gastric, liver, and colon cancer cell lines and in vivo with weak correlations to MYC amplification. TNBC and ovarian cancer immortalized and patient-derived cell lines indicated lower half-maximal inhibitory concentration values with increased MYC amplification or expression, although the results were not deemed significant. Fifteen patient-derived xenograft models used for in vivo assessments displayed a favorable correlation between tumor growth inhibition and MYC expression, including some tumor regressions in ovarian models.3
Moreover, treatment with KB-0742 was compared with 4 standard-of-care agents in 2 patient-derived organoid models of TNBC with differing treatment histories, and KB-0742 displayed significantly higher activity in the models, with maximal inhibition rates of 100% and 89%. In lymphoma-derived cell lines, treatment with the agent led to tumor growth inhibition of over 50%, including a tumor growth inhibition rate of 56% in a model of double-hit diffuse large B-cell lymphoma.3
“[KB-0742] has antitumor activity in TNBC, which is one of the targeted cancers for our expansion cohorts, and in NSCLC,” VillalonaCalero said. “[Its] activity and [how it] engages the target looked promising. [We have a couple of methods] for evaluating how it’s interacting with the target. One is looking at serine phosphorylation. We can also look at the downregulation of downstream target genes. Preclinically, the drug showed engagement of the target and activity when the target was engaged.”
Details of Phase 1/2 Trial of KB-0742
The phase 1/2 trial is the first-in-human study of KB-0742 and is looking to enroll a total of approximately 170 patients who are aged at least 12 years. Patients with relapsed/refractory solid tumors, including small cell lung cancer (SCLC), NSCLC, ovarian cancer, TNBC, and soft tissue sarcoma, and those with NHL will be eligible for enrollment. Patients must also have a body weight of at least 40 kg, an ECOG performance score of 1 or less, and adequate bone marrow and organ function. Those who previously underwent treatment with anticancer therapy within 4 weeks or 5 half-lives of enrollment, a history of surgery, a history of allogeneic transplant within 6 months of enrollment, and clinically significant heart disease will not be included in the study (Figure1).
Figure. Phase 1/2 Trial Design1
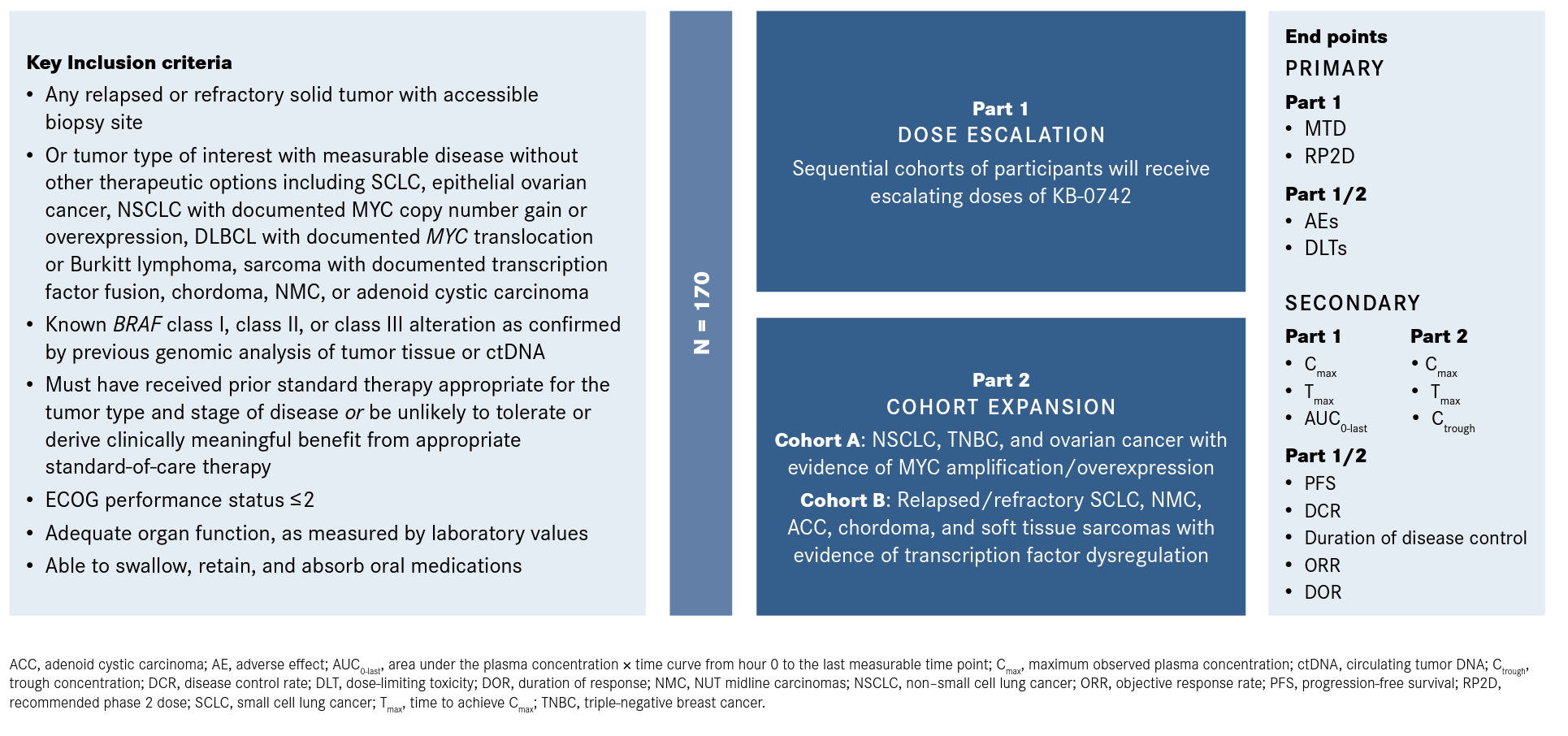
In part 1, the recommended phase 2 dose (RP2D) of KB-0742 was determined following the dose-escalation phase that started at 10 mg. KB-0742 was given weekly in a 3-days-on, 4-days-off manner. Patients in this part of the study were not selected for MYC amplification.4
In December 2022, the manufacturers of KB-0742 announced that the RP2D for part 2 of the study would be 60 mg. An analysis of available trial results found that the 60-mg dose led to a targeted reduction of approximately 50% in levels of phosphorylated Ser2 on RNA polymerase II, consistent with displaying antitumor activity based on preclinical models. KB-0742 was also found to have an acceptable safety profile, and the maximum tolerated dose (MTD) had not yet been identified.5
Additionally, among patients for whom pharmacokinetic data were available (n = 25), KB-0742 showed a terminal half-life of 24 hours, including an approximately 2.1- to 2.5-fold accumulation between day 1 and day 10. The agent displayed a differentiated pharmacokinetic profile, with oral bioavailability and dose-proportional exposure across all dose levels.
“We are almost there,” Villalona-Calero said. “We hit what we thought was a good dose that engaged with the target and moved forward into the expansion cohorts. However, we continued exploring a little more to see if we could give slightly higher doses. Nevertheless, the expansion cohorts are open and accruing at this point.”
In part 2, the cohort-expansion phase includes 2 cohorts that will enroll biomarker-selected patients who are deemed most likely to benefit from treatment with a CDK9 inhibitor. Cohort A will enroll approximately 40 patients with MYC-dependent solid tumors, including SCLC, NSCLC, TNBC, and ovarian cancer. In cohort B, approximately 30 patients with other transcriptionally addicted tumors, such as sarcoma and chordoma, will be enrolled.4
The primary end points in part 1 are determining the RP2D and the MTD. In parts 1 and 2, the primary end points are identifying the number of patients who experience dose-limiting toxicities and adverse events. Secondary end points include progression-free survival, disease control rate, overall response rate, and duration of response.1
The study is estimated to be completed in December 2025. Detailed findings from the dose-escalation stage and initial results from the phase 2 expansion cohorts are expected to be shared at a medical meeting in the second half of 2023.1,5
“We have a drug that is potentially active in many kinds of tumors,” Villalona-Calero said. “We would like to accrue more patients with TNBC into the trial. [In this trial], I will be concentrating on lung cancer, and other investigators who are collaborating with me are concentrating on sarcomas. Interestingly, sarcomas tend to occur in teenagers, and some trials do not allow [teenagers, as patients need to be] adults to participate. However, this trial allows patients to participate if they are 12 years or older. That’s another area we need to explore, and we welcome any referrals.”
References
- A dose escalation and cohort expansion study of KB-0742 in participants with relapsed or refractory solid tumors or non-Hodgkin lymphoma. ClinicalTrials.gov. Updated April 18, 2023. Accessed May 1, 2023. https://clinicaltrials.gov/ct2/show/NCT04718675
- CDK9 inhibitor KB-0742. National Cancer Institute. Accessed May 2, 2023. https://www.cancer.gov/publications/dictionaries/cancer-drug/def/cdk9-inhibitor-kb-0742?redirect=true
- Day MA, Saffran DC, Hood T, et al. KB-0742 is active in preclinical MYC high models of TNBC, ovarian, and DLBCL cancers. Cancer Res. 2022;82(suppl 12):2639. doi:10.1158/1538-7445.AM2022-2639
- Phase 1/2 study of KB-0742 in solid tumors. Kronos Bio. Accessed May 2, 2023. https://www.kronosbio.com/clinical-trials/phase-1-2-study-of-kb-0742-in-solid-tumors/
- Kronos Bio announces selection of recommended phase 2 dose for its oral CDK9 inhibitor, KB-0742, after reaching target engagement goal with acceptable safety profile in ongoing phase 1/2 clinical trial. News release. Kronos Bio. December 7, 2022. Accessed May 2, 2023. https://ir.kronosbio.com/news-releases/news-release-details/kronos-bio-announces-selection-recommended-phase-2-dose-its-oral









