Publication
Article
Supplements and Featured Publications
Pan-FGFR Inhibitor Seeks to Overcome Resistance Hurdles in Cholangiocarcinoma and Urothelial Carcinoma
Author(s):
Early sensitivity to FGFR inhibition has improved outcomes for patients across tumor histologies; however, kinase domain mutations limit extended efficacy for select patients including those with intrahepatic cholangiocarcinoma or urothelial cancer.
Mitesh J. Borad, MD

Early sensitivity to FGFR inhibition has improved outcomes for patients across tumor histologies; however, kinase domain mutations limit extended efficacy for select patients including those with intrahepatic cholangiocarcinoma or urothelial cancer. Primary and acquired mutations associated with clinical resistance highlight the need for therapeutic development to focus on preventing the activation of these oncogenic driver alterations.1,2
“Alterations in the FGFR pathway are prevalent in many solid tumors, [as well as] some hematological malignancies,” Mitesh J. Borad, MD, said in an interview with OncologyLive®. “One cancer where [FGFR2 fusions are] more prevalent [include] intrahepatic cholangiocarcinoma, where the prevalence is estimated in the 10% range in the intrahepatic subtype. There are also FGFR2 mutations, which are found in [approximately] 3% to 4% of [tumors], mostly in the intrahepatic [subtype]. In bladder cancer, the alterations are mostly with FGFR3, and they’re most commonly mutations in exon 7 and exon 10.” Borad noted that there have been varying estimates reported in the 20% to 40% range depending on the subset of bladder cancer. Borad is a medical oncologist at Mayo Clinic Comprehensive Cancer Center in Phoenix, Arizona.
Identifying patients with these mutations has presented significant improvements in clinical outcomes, according to Sameek Roychowdhury, MD, PhD.
“When we identify gain-of-function, activating FGFR alterations, whether it be a fusion or a point mutation in cholangiocarcinoma or bladder cancer, that helps us identify a subset of patients who are likely to benefit from an FGFR-targeted kinase inhibitor,” Roychowdhury said in an interview with OncologyLive®, adding that patients without the mutations may not experience any benefit. Roychowdhury is an assistant professor in the Department of Internal Medicine and the Department of Pharmacology at The Ohio State University in Columbus.
Patients with intrahepatic cholangiocarcinoma or urothelial carcinoma represent 2 populations with approved FGFR inhibitors targeting FGFR2 and FGFR3, respectively.3-5 Approvals of agents such as infigratinib (Truseltiq) and pemigatinib (Pemazyre) for patients with cholangiocarcinoma with an FGFR2 rearrangement or fusion have shown overall response rates of 23% and 36%, respectively, with median durations of response ranging from 5 to 9 months, respectively.3,4 Nearly all patients (98%) treated had intrahepatic cholangiocarcinoma.
Erdafitinib (Balversa) in advanced or metastatic urothelial carcinoma with FGFR3 or FGFR2 alterations demonstrated an overall response rate of 40% with a median duration of 5.6 months according to updated data from the BCL2001 trial (NCT02365597) published in Lancet Oncology.6
Although overall response rates are below 40% for the approved indications, clinical benefit has been seen in other end points. “In cholangiocarcinoma, we are seeing anywhere from 70% to 80% of patients having some kind of clinical benefit,7 whether it’s a partial response or stable disease, they’re experiencing disease control. In bladder cancer, we’re seeing a benefit rate of approximately 40%,6 where patients are having partial responses and stable disease.”
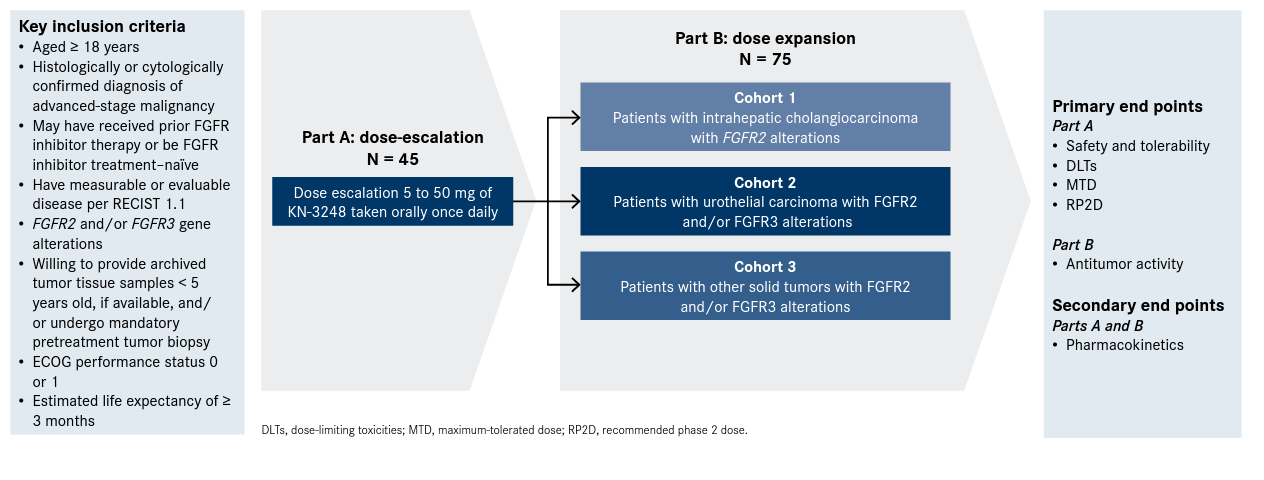
Figure. Phase 1 Trial Design for FGFR2 or FGFR3 Mutant Solid Tumors2,10
Following the Mutation Trail
Primary and acquired mutations observed with first-generation FGFR inhibitors have prompted investigators to pinpoint opportunities to react or circumvent their development. “Just like we’ve seen in lung cancer, where you start with the first-generation inhibitors, you understand the mechanisms of resistance, and then subsequently better drugs are developed and you can prolong that response or you can [begin to] sequence drugs as newer generation inhibitors are brought through the development pipeline,” Borad said. “What we understand with FGFR2 and FGFR3 is that the mechanisms of resistance can be on pathway or off pathway. When they’re on pathway, they may be mutations in FGFR2 or FGFR3 themselves, often polyclonal mutations where you have many clones in the tumor, each having different mutations. You can often determine this is happening by liquid biopsy analysis. When [this occurs] you need drugs that can cover a lot of these different mutations because there’s multiple mechanisms of pathway resistance.”
Gatekeeper and molecular brake mutations, such as V565F and N550H, respectively, have demonstrated an ability to escape inhibition with available FGFR inhibitors. Further, acquired bypass resistance mechanisms result in limited long-term efficacy for patients with cholangiocarcinoma and urothelial cancer.8
“The average time to progression is approximately 6 months,” Roychowdhury said. “The [emerging] mutations tend to interfere with how the [FGFR inhibitor] interacts with the kinase part of the receptor. But we’ve also seen other mechanisms [arise]—mutations and other genes and pathways—that tend to be downstream of the receptor. We often see evidence of MAPK or PI3K activation downstream after FGFR resistance develops.”
Overcoming the Resistance Hurdle
An all-encompassing approach to blocking outgrowth activity of FGFR resistance clones and mutations, investigators have initiated a phase 1 study (NCT05242822) to evaluate the preliminary efficacy of KIN-3248, a next-generation pan-FGFR inhibitor in individuals with advanced solid tumors harboring FGFR2 and/or FGFR3 gene alterations.2 The first patient in the trial was dosed in April 2022.9
KIN-3248 was designed to treat patients with intrahepatic cholangiocarcinoma, UC, and other solid tumors. In preclinical studies, KIN-3248 demonstrated activity across a range of mutations that drive primary disease and acquired resistance to other FGFR inhibitors. For example, the agent has shown that it can inhibit the activation of the FGFR-MAPK pathway in cells that express FGFR2 gatekeeper mutations.2,8
KN-4802 is a multicenter, open-label, 2-part study that aims to enroll approximately 120 patients with advancedstage solid tumors.2,10 Patients must have confirmed FGFR2 and/ or FGFR3 mutations by previous genomic analysis of tumor tissue or circulating tumor DNA. Patients can be naïve to, or pretreated with, FGFR inhibitors.
“With targeted therapies, you want to focus on those patients who have these alterations and keep [the populations] somewhat broad during dose escalation to arrive at the doses you would use in phase 2 studies and potentially pivotal studies,” Borad explained of the primary solid tumor population. “There are expansion cohorts in intrahepatic cholangiocarcinoma, where you have the FGFR2 events and urothelial cancer, where you have the FGFR3 events—predominantly mutations—and then a category that would encompass other solid tumors where prevalence may be in the low percentage range.”
Part A will determine the recommended dose and will include 6 dose levels: 5 mg, 10, mg, 20 mg, 30 mg, 40, mg, and 50 mg. KIN-3248 is taken orally once daily.2
Once the maximum tolerated dose (MTD) is established, the dose-expansion phase of the trial will examine the safety and efficacy of the agent.2
Primary end points in the dose-escalation portion include incidence of DLTs and to identify the MTD and/or recommended phase 2 dose (RP2D), with secondary end points including pharmacokinetics assessing maximum plasma concentration. Once the MTD and/or RP2D is established, the dose-expansion phase of the trial will examine the safety and efficacy of the agent.2,10
Primary end points in the dose-expansion part include objective response rate, disease control rate, duration of response, and progression-free survival. Exploratory end points include overall survival, exposure-safety and exposure-efficacy relationships, and durability of stable disease.
Patients must have measurable disease per RECIST 1.1 criteria, an ECOG performance status of 0 or 1, and adequate organ function to enroll. Patients with clinically active or clinically progressive brain metastases are not eligible for enrollment.2,10
Roychowdhury noted that “paying close attention to FGFR alterations” is a key takeaway for clinicians seeing patients with cholangiocarcinoma or urothelial carcinoma. “There are some known gain-of-function fusions and mutations, but I think we’re beginning to see that there are other gain-of-function mutations that are not that common,” he said. “Whenever you do see an FGFR mutation, look closely to see if you can get the match to a clinical trial that accepts that type of mutation. Keep an eye out for these patients.”
The trial is open to enrollment.
References
- Krook MA, Reeser JW, Ernst G, et al. Fibroblast growth factor receptors in cancer: genetic alterations, diagnostics, therapeutic targets and mechanisms of resistance. Br J Cancer. 2021;124(5):880-892. doi:10.1038/s41416-020-01157-0
- Goyal L, Perez CA, Kato S, et al. Design and rationale of a f irst-in-human (FIH) phase 1/1b study evaluating KIN-3248, a next-generation, irreversible (irrev), pan-FGFR inhibitor (FGFRi), in adult patients with solid tumors harboring FGFR2 and/or FGFR3 gene alterations (NCT05242822). J Clin Oncol. 2022;40(suppl 16):TPS9601. doi:10.1200/JCO.2022.40.16_suppl.TPS9601
- FDA grants accelerated approval to infigratinib for metastatic cholangiocarcinoma. FDA. May 28, 2021. Accessed August 31, 2022. bit.ly/3eiyI7J
- FDA grants accelerated approval to pemigatinib for cholangiocarcinoma with an FGFR2 rearrangement or fusion. FDA. Updated April 20, 2020. Accessed August 31, 2022. bit.ly/3RpMdAS
- FDA grants accelerated approval to erdafitinib for metastatic urothelial carcinoma. April 12, 2019. Accessed August 31, 2022. bit.ly/3R6elcy
- Javle MM, Roychowdhury S, Kelley RK, et al. Final results from a phase II study of infigratinib (BGJ398), an FGFR-selective tyrosine kinase inhibitor, in patients with previously treated advanced cholangiocarcinoma harboring an FGFR2 gene fusion or rearrangement. J Clin Oncol. 2021;39(suppl 3):265. doi:10.1200/ JCO.2021.39.3_suppl.265
- Siefker-Radtke A, Necchi A, Park SH, et al; BCL2001 Study Group. Efficacy and safety of erdafitinib in patients with locally advanced or metastatic urothelial carcinoma: long-term follow-up of a phase 2 study. Lancet Oncol. 2022;23(2):248-258. doi:10.1016/S14702045(21)00660-4
- Franovic A, Mohan A, Uryu, et al. Activity of KIN-3248, a next-generation pan-FGFR inhibitor, against acquired FGFR-gatekeeper and molecular-brake drug resistance mutations. J Clin Oncol. 2022;40(suppl 4):461. doi:10.1200/JCO.2022.40.4_suppl.461
- Kinnate Biopharma Inc. announces first patient dosed in phase 1 clinical trial of its FGFR inhibitor product candidate, KIN-3248. News release. Kinnate Biopharma Inc. April 18, 2022. Accessed September 1, 2022. bit.ly/3wRTlhD
- A study to evaluate KIN-3248 in participants with advanced tumors harboring FGFR2 and//or FGFR3 gene alterations. ClinicalTrials.gov. Updated August 11, 2022. Accessed September 1, 2022. https://clinicaltrials.gov/ct2/show/ NCT05242822