Publication
Article
Contemporary Radiation Oncology
Prognostic Significance of Post-Treatment PET/CT Following Salvage Re-irradiation of Head and Neck Cancers
Author(s):
Researchers explored the prognostic significance of PET/CT following re-irradiation in patients with head and neck cancer.
About the lead author:
Tawee Tanvetyanon, MD, MPH
Moffitt Cancer Center
Tampa, FL
Expert’s Perspective
Megan E. Daly, MD
Department of Radiation Oncology
UC Davis Medical Center
Why is this article contemporary?
Re-irradiation is an increasingly employed strategy for locally recurrent head and neck cancer, and in select cases may result in long-term disease-free survival. However, disease surveillance following re-irradiation poses several unique challenges. Following de novo radiation for primary head and neck cancer, the utility of imaging modalities that may augment physical exams is well-studied. Positron emission tomography and computed tomography (PET/CT) scanning has a negative predictive value in excess of 90% in this setting, and provides valuable prognostic information to patients and physicians. Although it is tempting to extrapolate these results, the increased tissue inflammation and necrosis, and the increased risk of distant metastases following re-irradiation, could complicate imaging interpretation, and the performance of PET/CT following re-irradiation has not been thoroughly evaluated.
In the present study, the authors explore the prognostic significance of PET/CT following re-irradiation in a cohort of recurrent head and neck cancer patients. They identify a positive predictive value for PET/CT of >95%, suggesting PET/CT may be a valuable component of surveillance in this setting. Evidence-based surveillance algorithms are lacking for many cancer types, and in an era of value-based care, well-designed studies that demonstrate the value of specific imaging studies are needed. This study is an important first step in assessing the utility of PET/CT following re-irradiation for recurrent head and neck cancer.
Abstract
Objective
Positron emission tomography/computed tomography (PET/CT) has been widely used to assess tumor response following chemoradiotherapy of head and neck cancer. In general, a negative post-treatment PET/CT is associated with a good prognosis. Nevertheless, in the setting of re-irradiation, an increasingly adopted salvage treatment modality, this association remains unclear. In this report, we explored the prognostic significance of PET/CT following re-irradiation.
Materials and Methods
The records of patients who underwent re-irradiation for recurrent or new primary head and neck cancer as well as PET/CT scans before and at 1.5 to 4.0 months after treatment were reviewed. Re-irradiation was given with intensity-modulated radiation therapy at a median dose of 60 GY with a cumulative dose of 130 GY. Concurrent chemotherapy was given in all but three patients. Semi-quantitative analyses of PET/CT were re-performed. Metabolic response was assessed using PET response criteria in solid tumors (PERCIST) criteria.
Results
Included for analyses were 54 patients; 16 patients (30%) had a negative post-treatment PET/CT and 38 patients (70%) had a positive scan. At a median follow-up time of 55 months, 44 patients had died. Among those with negative PET/CT, the median overall survival was 38.7 months, compared with 9.4 months among those with positive PET/CT (P <.001), corresponding to estimated 5-year survival rates of 42.2% and 3.6%, respectively. The positive predictive value of PET/CT for 5-year mortality was 96.4%, while the negative predictive value was 42.2%. By PERCIST criteria, a statistically significant survival advantage was observed only among patients with a complete metabolic response, but not partial metabolic response.
Conclusions
Based on our limited experiences, PET/CT after re-irradiation can be useful for prognostic purposes. Although a negative post-treatment PET/CT may not reliably portend a good prognosis, a positive PET/CT practically rules out the possibility of long-term survival.
Introduction
Re-irradiation is a well-recognized salvage therapy for head and neck cancer patients who develop recurrent disease or new primary cancer in a previously irradiated area. In the absence of distant metastatic disease, about 10 to 20% of such patients may become long-term survivors after re-irradiation, with or without salvage surgery.1-3 Data from a randomized phase III study has shown that, after a salvage surgery, treatment with adjuvant re-irradiation along with concurrent chemotherapy improves progression-free survival (PFS) when compared with observation alone.4 For this clinical scenario, therefore, the National Comprehensive Cancer Center Guideline currently recommends that re-irradiation be considered as one of the limited treatment options including additional surgery, palliative chemotherapy, or best supportive care.5
Nevertheless, there remains a paucity of published experiences on the prognostication of patients following this treatment. While some clinical factors such as interval since previous radiation, tumor bulk at re-irradiation, salvage surgery, nodal disease, comorbidity, and organ dysfunction may help estimate the likelihood of long-term survival,6 this prognostic schema does not take into account the value of tumor response after re-irradiation.
During the past decade, an imaging modality with [18F]-fluorodeoxyglucose (18F-FDG) positron emission tomography (PET) integrated with computerized tomography (PET/CT) has been increasingly used for tumor staging and radiotherapy planning, as well as tumor response assessment.7,8 Specifically, in the tumor response assessment of head and neck cancer patients following chemoradiation, PET/CT is highly prognostic. A negative PET/CT indicating a complete metabolic response (CMR) has been associated with an absence of future disease recurrence with a negative predictive value—the probability that a person who is a test negative is a true negative— typically over 90%.9-11
While the usefulness of post-treatment PET/CT has been well demonstrated among first-time irradiated patients, this has not been systematically investigated among re-irradiated patients. In the re-irradiation setting, some unique issues that may affect the accuracy of PET/CT will need to be considered. First, in the re-irradiation setting, there is generally a heightened prevalence of tissue necrosis, infection, or aspiration, which can cause tissue hypermetabolism, thereby causing a false-positive finding on PET/CT.12,13 Second, due to a partial-volume effect, PET/ CT may not reliably detect sub-centimeter lesions.14,15 This is especially relevant because, in the re-irradiation setting, there may be an increased prevalence of small-volume metastatic disease, considering the aggressive biology of the recurrent cancer and the fact that pre-treatment scans have already screened out patients with obvious metastatic disease from being candidates for re-irradiation.
Methods and Materials
Study Sample
Radiotherapy and Chemotherapy
18F-PET/CT Protocol
Response Assessment
Statistical Analysis
Given these considerations, we sought to investigate the prognostic significance of PET/CT scans in the salvage re-irradiation setting. Our primary objective was to evaluate long-term 5-year survival based on the response observed from post-treatment PET/CT scans. Our secondary objectives were to describe the degree to which metabolic responses by PET response criteria in solid tumors (PERCIST) criteria among a subgroup of patients with gross disease at the start of re-irradiation predicted survival, and to evaluate if the prognostic significance of PET/CT will remain significant and independent from other clinical factors that have been already recognized as survival predictors after salvage re-irradiation therapy.After approvals from the Institutional Scientific Review Committee and Institutional Review Board, eligible patients with locally recurrent squamous cell carcinoma of the head and neck treated with definitive re-irradiation during January 2005 to 2012 with a curative intent were retrospectively identified. For patients who underwent salvage surgery, at least one of the following high-risk pathological features must have been present: positive surgical margins, multiple lymph node involvement, perineural invasion, or soft tissue invasion. Re-irradiation was defined as a significant (>50%) overlap of the previously irradiated field. Human papilloma virus (HPV) testing was performed among select patients with oropharygeal primary using p-16 immunohistochemistry or Fluorescence in-situ hybridization in which positivity was based on diffuse and intense reaction. Staging was per the American Joint Committee on Cancer 7th edition.16 Comorbidity was assessed by Charlson’s index.17 Organ dysfunction was defined as feeding tube dependency, functioning tracheostomy, or soft tissue defect including history of osteonecrosis. Prophylactic feeding tube placement before re-irradiation was not considered as organ dysfunction. This study included the previously reported outcomes from nine patients.6Re-irradiation was confined to the local relapse region (no elective uninvolved lymph node included) and was given as 2-Gy fraction daily using linear accelerator with intensity modulated radiation (IMRT) technique. Target tumor volume was planned based on computed tomography (CT) scans. In addition, for patients with gross disease, PET/CT images were fused with planning CT images to delineate the area of 18F-FDG avidity. Gross tumor volume was first derived and a margin of 5 mm each was added for clinical target volume and planning target volume with appropriate reduction to avoid critical structure. Concurrent chemotherapy or immunotherapy was prescribed as followed: cisplatin was given as 75-100 mg/m2 every 3 weeks or 25- 30 mg/m2 weekly; carboplatin was given as AUC 5-6 every 3 weeks or AUC 1-2 weekly; and cetuximab was given as a 500 mg/m2 loading dose one week before re-irradiation, then 250 mg/ m2 weekly. All chemotherapy was discontinued at the completion of re-irradiation. Pre-treatment tumor size was measured on contrasted CT scans. Tumor burden reflected the sum of the maximal diameter of the primary tumor plus the diameter of cervical adenopathy. For patients with no gross disease before re-irradiation, their tumor burden was recorded as zero.Patients underwent PET/CT within 4 months after completion of re-irradiation. In addition, among those with gross disease before re-irradiation, pre-treatment PET/CT was performed for staging purposes before re-irradiation. At our institution, patients were scanned on a Biograph PET system (Siemens Medical Solutions) or Discovery VCT PET/CT system (GE Medical Systems), maintained to manufacturer specification. All patients underwent overnight fasting, and serum glucose below 200 mg/dl was confirmed prior to imaging. PET/CT image acquisition occurred at approximately 90-120 minutes after 18F-FDG administration at 3 MBq/kg (81 μCi/kg) body by intravenous injection. PET emission data were obtained in 3D mode for two minutes at each bed position. CT studies for attenuation correction and anatomic co-registration were performed without contrast in a low dose protocol (30 mA, 120 kV) covering the area from base of the skull to the proximal thighs. Quantification at review was performed on images compliant with the Digital Imaging and Communication in Medicine standard, using ADW 4.2 (GE Medical Systems) processing software.18 Available digitized images of fused PET/CT scans were reviewed by one nuclear medicine physician who was unaware of patient clinical outcome. Semi-quantitative evaluation was based on maximum standardized uptake value (SUVmax) calculated as the ratio of measured activity concentration over an assumed homogeneous distribution of the applied radioactivity19 on the spherical region of interest at the most intense lesion.The SUVmax was used as a measurement index, measured using volumetric region-of-interest technique with standard image analysis software. Metabolic response was assessed according to PERCIST version 1.0.20 CMR or negative PET/CT was defined as a complete resolution of 18F-FDG uptake within the target lesion rendering it indistinguishable from surrounding background blood-pool levels with no new 18F-FDG-avid lesions. Partial metabolic response was a reduction of a minimum of 30% in the target tumor standardized uptake value (SUV). Progressive metabolic disease is a 20% increase in SUV or emergence of new 18F-FDG-avid lesions consistent with neoplasm. For the purpose of graded PERCIST response category, only patients with available baseline PET/CT performed within 3 months before the beginning of re-irradiation were included and for those with multiple PET/CT during the period, only values obtained from the test performed closest to the re-irradiation initiation was used.Descriptive statistics, including median and range for continuous variables and frequency and percentage for categorical variables, were obtained. Pearson’s Chi-square or Fisher’s exact test were used to compare categorical variables with Monte Carlo estimation as appropriate. To address a non-normal distribution, the non-parametric Mann-Whitney test was used to compare duration from previous re-irradiation and tumor burden between groups.
Results
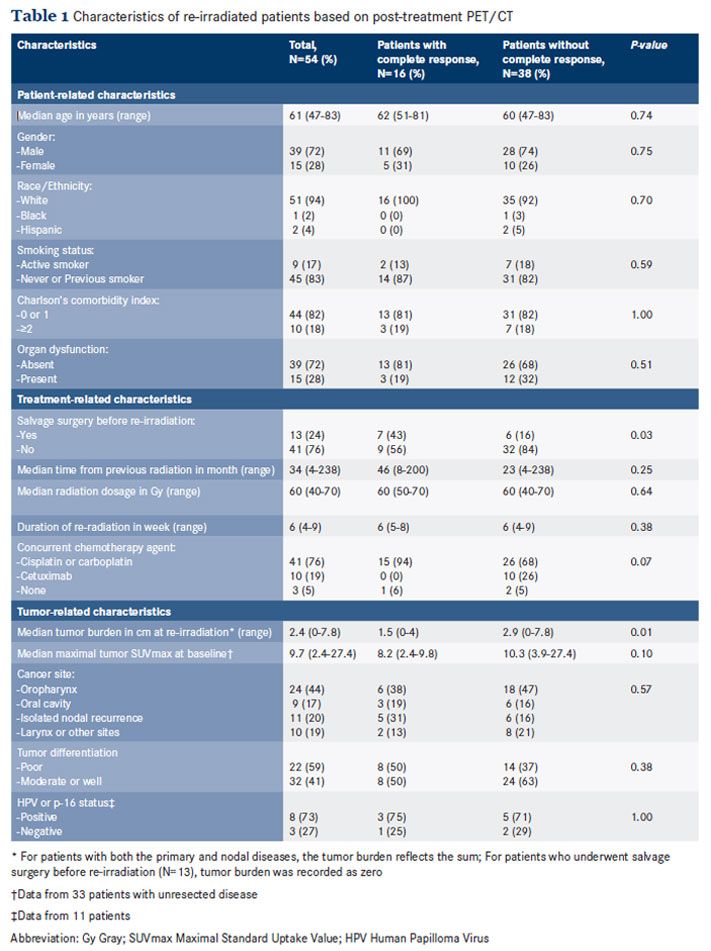
Overall survival (OS) was calculated from re-irradiation until death or last follow-up and PFS was from re-irradiation until death, progression, or last follow-up, whichever occurred first. Death was verified by the National Social Security Death Index. Survival estimates were obtained by the method of Kaplan-Meier. The point estimates of survival were used to compute the performance characteristics of PET/CT in predicting disease progression or death at 2 and 5 years. The log-rank test was used to compare the survival between groups. The Cox-proportional hazards regression model was fitted to examine the independent impact of CMR on survival outcomes, adjusted for other prognostic factors. All P-values were 2-tailed and the significant level was set at P <0.05. All analyses were performed on SAS version 9.4 (Cary, NC) or SPSS version 22 (Chicago, IL).Patient, Treatment, and Tumor Characteristics Data from 54 patients were analyzed. On the basis of post-treatment PET/CT, 16 patients (30%) achieved CMR and 38 patients (70%) failed to achieve CMR. The characteristics of patients with or without CMR were comparable (Table 1). The majority of patients in both groups were males who had no active smoking history or significant comorbidity. Organ dysfunction was present at baseline in 15 patients (28%): Feeding tube dependency was observed in 11 patients, functioning tracheostomy in 4 patients (2 patients were also feeding tube-dependent), and osteonecrosis in 2 patients.
For treatment-related characteristics, salvage surgery occurred in 13 patients (24%) prior to re-irradiation. A higher proportion of patients who underwent salvage surgery achieved CMR than those with gross tumors at re-irradiation: 43% versus 16%, P = 0.03. There was a higher proportion of cetuximab usage observed in the group of patients without CMR: 10 patients versus zero patient, P = .07. In both groups, the median time interval between the initial and present radiotherapy was 3 years and the dosage or duration of re-irradiation was comparable.
Post-Treatment PET/CT and Survival
With regard to tumor-related characteristics, the overall median tumor burden before re-irradiation was 2.4 cm. Consistent with a higher proportion of patients who underwent salvage surgery being in the CMR group, lower tumor burden was also observed in the CMR group: 1.5 cm versus 2.9 cm, P = .01. In both groups, the most common disease sites were the oropharynx and oral cavity. HPV or p-16 staining was performed in 11 patients; 8 patients (73%) tested positive.There were 13 patients (24%) whose physical examination was suspicious for residual disease. Post-treatment PET/CT of 54 patients was performed at a median of 2.9 months (range 1.5 to -4.0 months) after re-irradiation completion. The median acquisition time of PET/CT was 1.8 hours. The observed median SUVmax was 5.9.
CMR was observed in 16 patients (30%) and failure to achieve CMR occurred in 38 patients (70%). Among the 38 patients who failed to achieve CMR, 30 patients (80%) had persistent hypermetabolism in the treatment field and 8 patients (20%) developed a new site of disease outside the treatment field, including 3 patients with distant metastases. To date, death had occurred in 44 patients, corresponding to a median OS of 14 months (95% CI: 11.4-16.5). The median follow-up time was 55.4 months (range 7.5-96.1). Progressive disease or death had occurred among 46 patients, corresponding to a median PFS of 7.2 months (95% CI: 4.5-9.9). The estimated 5-year OS and PFS rates were 15.7% and 13.2%, respectively.
Prognostic Significance of PET/CT in the Context of Other Variables
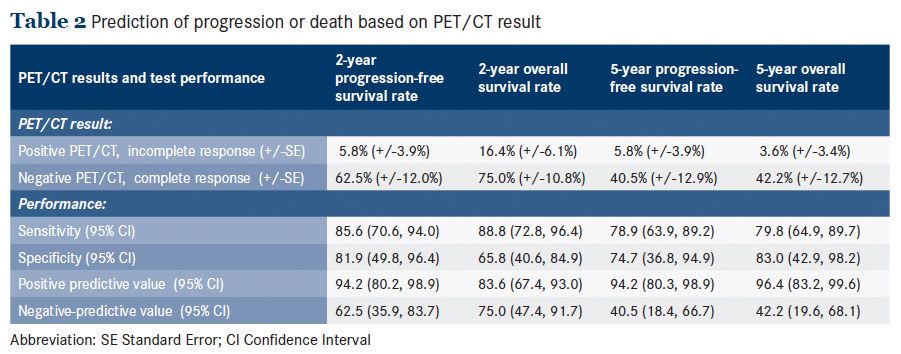
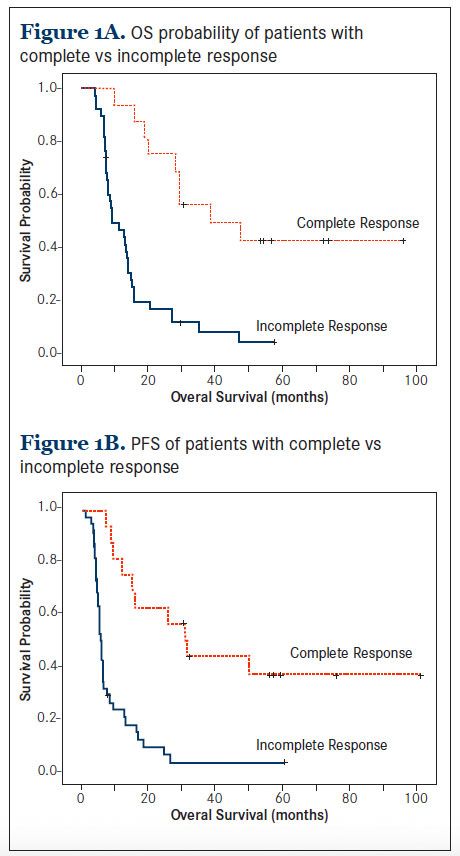
When considering survival by post-treatment PET/CT results, patients who achieved CMR had a significantly better median OS than those who did not: 38.7 months versus 9.4 months, P <.001 (Figure 1A). This corresponded to estimated 2-year OS rates of 75% versus 16.4% and 5-year OS rates of 42.2% versus 3.6%, respectively. In addition, patients who achieved CMR had a significantly better PFS than their counterparts: 29.2 months versus 5.6 months, P <.001 (Figure 1B). This corresponded to estimated 2-year PFS rates of 62.5% versus 5.8% and 5-year PFS rates of 40.5% versus 5.8%, respectively. Based on these estimates, the performance of PET/CT in predicting survival outcomes at 2- and 5-year time points were calculated (Table 2). Positive PET/CT predicted progression or death with higher positive predictive values than negative predictive values. The positive predictive value—the probability that a person who is a test positive is a true positive— ranged from 83.6% to 96.4%, while the negative predictive value— the probability that a person who is a test negative is a true negative—ranged from 40.5% to 75%.We evaluated the predictive value of PET/CT on survival in the context of other prognostic factors (Table 3). In a univariable analysis, positive PET/CT was associated with an increased risk of death with % ratio (HR) 4.9 (95% CI: 2.3, 10.5) and an increased risk of progression or death with HR 4.9 (2.3, 10.3).
Change in Tumor Metabolism and Survival
In a multivariable analysis incorporating other known prognostic factors of re-irradiation, positive PET/CT remained predictive of overall mortality with HR 7.2 (95% CI: 2.7-19.6) and of progression or death with HR 6.8 (95% CI: 2.6-17.5, P <0.001). In this multivariable model, the second best predictor of survival was the lack of salvage surgery, with HR 3.97 (0.77, 20.41), though this association did not reach statistical significance (P = 0.10).We performed a subgroup analysis among patients who did not undergo salvage surgery. Of these, there were 33 patients with available baseline PET/CT, thus allowing for a graded metabolic response assessment based on the PERCIST percentage change in the tumor metabolism.
Among these patients, their pre-treatment PET/CT scans were performed at a median of 2.1 weeks (range 1 to 11.8) prior to re-irradiation. The median acquisition time of these scans was 1.9 hours (range 1.3 to 3.7). The median pre-treatment SUVmax was 9.7 (range 2.4 to 27.4). After re-irradiation, the median SUVmax was 6.1 (range 0 to 15.7). This change in tumor metabolism was equal to a median reduction of 46% (range -86% to +100%) (Figure 2). There were 7 patients with higher-than-baseline tumor metabolism and 6 of them met the criteria for progressive disease. According to PERCIST, 8 patients (24%) achieved CMR, 14 patients had a partial metabolic response (42%), 5 patients (15%) had a stable metabolic disease, and 6 patients (18%) developed progressive metabolic disease.
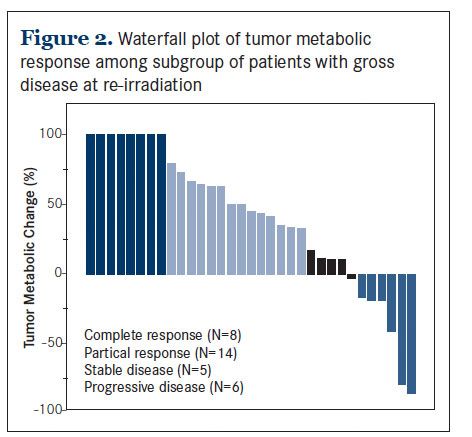
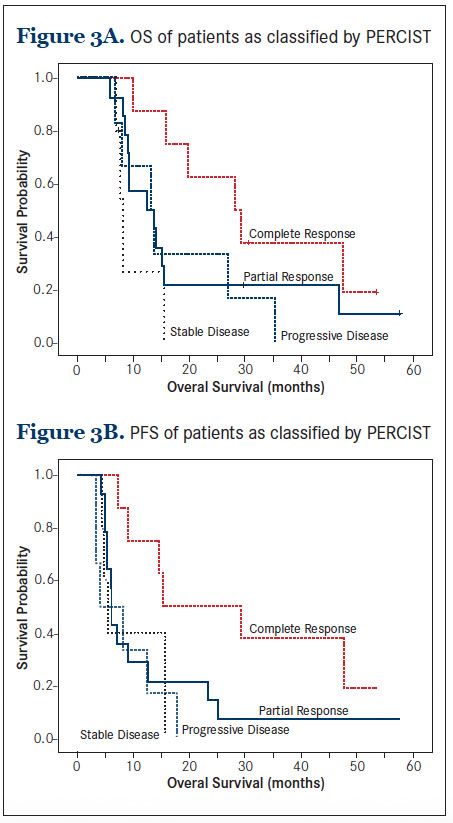
To explore the association between tumor response and OS, we compared the survival outcomes among the 4 patient groups classified by PERCIST category. While patients with CMR emerged as the group with the best survival outcome, there was little difference in the survival among those with partial metabolic response, stable metabolic disease, or progressive metabolic disease (Figure 3A).
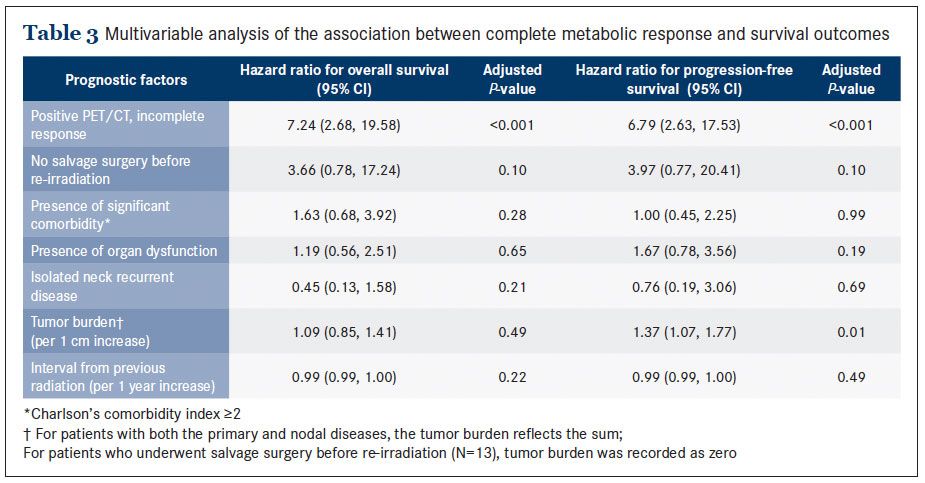
Discussion
This pattern of association was also observed with the PFS outcome (Figure 3B). Overall, when comparing patients with and without CMR, those with CMR had significantly better survival outcomes. The median OS was 28.2 months (95% CI: 15.2 to 41.3) among those with CMR, compared with 13.2 months (95% CI: 7.9 to 18.5) among those without CMR, respectively (P = 0.034), and the median PFS was 15.4 months (95% CI: 5.1 to 11.3), compared with 6.1 months (95% CI: 4.9 to 7.2), respectively (P = 0.015).In this study we reported on the utility of PET/CT in the salvage re-irradiation for head and neck cancer. We found that CMR, a negative post-treatment PET/CT, occurred in only 30% of patients. CMR was not strongly predictive of long-term survival; however, failure to achieve CMR was strongly predictive of disease progression or death. This observation remained robust even after accounting for other known prognostic factors including salvage surgery. Overall, failure to achieve CMR projected about a 7-fold increase in the risk of death or progression. Therefore, the potential clinical utility of PET/CT in the re-irradiation setting appears to lie in its high positive predictive value.
In contrast to the observation derived from the chemoradiation among radiation-naïve patients, a negative post-treatment PET/CT in the re-irradiation setting does not necessarily portend a good prognosis. This likely reflects the natural history of patients who develop recurrent or new primary head and neck cancer in that they are more likely to have future recurrence or may develop complications that can lead to an increased mortality even if disease is initially controlled. This observation suggests that patients with a negative post-treatment PET/CT will still need to be considered for an intensive follow-up. Furthermore, for patients with positive post-treatment PET/CT, our limited experiences indicate that they will likely develop disease progression or death. For such patients, additional treatment should be considered if deemed appropriate.
While there appears to be a distinction in how PET/ CT can be applied in clinical practice between the radiation- naïve setting and the re-irradiation setting, the sensitivity and specificity—which are intrinsic qualities of the test—of PET/CT still remain quite comparable. In previously reported series, the sensitivity and specificity of PET/ CT to predict survival in the radiation-naïve setting are, respectively, in the range of 70 to 80% and 80 to 90%.9-11 In our study, both the sensitivity and specificity estimates are also in the 70 to 80% range, depending on the referenced survival time point. The key difference between the two settings, however, is the prevalence of outcome; that is recurrence or death, which is higher in the re-irradiation setting than in the initial radiation setting. For instance, the estimated 5-year OS rate in our study was only 16%, compared to 60 to 70% in the first-time radiation setting.9-11 Such high prevalence of outcome, therefore, translates into the high positive predictive value of positive post-treatment PET/ CT observed in the current study.
Although, to our knowledge, this is the only report of PET/CT utility in the salvage re-irradiation setting with semi-quantitative re-analysis, a number of limitations should be considered. First, due to a small sample size, there may not be adequate statistical power to detect any survival difference by PERCIST response category, specifically to detect the survival advantage associated with partial metabolic response. Second, there was a variation in the timing of the PET/CT performance ranging from two to four months after re-irradiation. Although the optimal timing of post-treatment PET/CT remains to be defined, false positivity and false negativity rates may be affected by the timing of PET/CT. Finally, in our practice, patients with an obvious clinical progression of disease may not necessarily undergo post-treatment PET/CT, and therefore patient selection to undergo the scan can affect the positive or negative predictive values of PET/CT. Although the vast majority of the PET/CT scans were done for surveillance purposes, some scans were performed in an attempt to differentiate between residual disease and radiation-induced tissue necrosis. Due to the retrospective nature of this study, we were unable to confirm the intent of PET/CT scan in all cases.
In summary, our experiences herein illustrate the prognostic significance of 18F-FDG PET/CT scan in the salvage re-irradiation setting. Failure to achieve CMR is highly predictive of disease progression or death. In addition, this prognostic significance of PET/CT is superior to other known pre-treatment prognostic indicators. A study with a larger sample size will be needed to understand the prognostic significance by PERCIST criteria.
About the Authors
Department of Head and Neck Oncology (TT, TP, JM, JK, JC, AT), Department of Thoracic Oncology (TT), Department of Radiation Oncology (JC, AT), Department of Diagnostic Imaging (EE), University of South Florida, H. Lee Moffitt Cancer Center and Research Institute, Tampa, FL
Address correspondence to: Tawee Tanvetyanon, MD, MPH, 12902 University of South Florida Magnolia Dr., Tampa, FL 33612. Phone: 813-745-7640, Fax: 813-745-3027. E-mail: tanvett@moffitt.org
Disclosures: None.
Conflicts of interest: None.
References
- Lee N, Chan K, Bekelman JE, Zhung J, Mechalakos J, Narayana A, et al. Salvage re-irradiation for recurrent head and neck cancer. Int J Radiat Oncol Biol Phys. 2007;68(3):731-740
- Spencer SA, Harris J, Wheeler RH, Machtay M, Schultz C, Spanos W, et al. Final report of RTOG 9610, a multi-institutional trial of reirradiation and chemotherapy for unresectable recurrent squamous cell carcinoma of the head and neck. Head Neck. 2008 Mar;30(3):281-288.
- De Crevoisier R, Bourhis J, Domenge C, Wibault P, Koscielny S, Lusinchi A, et al. Full-dose reirradiation for unresectable head and neck carcinoma: experience at the Gustave-Roussy Institute in a series of 169 patients. J Clin Oncol. 1998 Nov;16(11):3556-62.
- Janot F, de Raucourt D, Benhamou E, Ferron C, Dolivet G, Bensadoun RJ, et al. Randomized trial of postoperative reirradiation combined with chemotherapy after salvage surgery compared with salvage surgery alone in head and neck carcinoma. J Clin Oncol. 2008 Dec 1;26(34):5518-5523.
- Pfister DG, Ang KK, Brizel DM, Burtness BA, Busse PM, Caudell JJ, et al. National Comprehensive Cancer Network. Head and neck cancers, version 2.2013. Featured updates to the NCCN guidelines. J Natl Compr Canc Netw. 2013 Aug;11(8):917-923.
- Tanvetyanon T, Padhya T, McCaffrey J, Zhu W, Boulware D, Deconti R, et al. Prognostic factors for survival after salvage reirradiation of head and neck cancer. J Clin Oncol. 2009 Apr 20;27(12):1983-1991.
- Fortin D, Basran PS, Berrang T, Peterson D, Wai ES. Deformable versus rigid registration of PET/CT images for radiation treatment planning of head and neck and lung cancer patients: a retrospective dosimetric comparison. Radiat Oncol. 2014 Feb 10;9:50.
- Vernon MR, Maheshwari M, Schultz CJ, Michel MA, Wong SJ, Campbell BH, et al. Clinical outcomes of patients receiving integrated PET/CT-guided radiotherapy for head and neck carcinoma. Int J Radiat Oncol Biol Phys. 2008 Mar 1;70(3):678-684
- Sherriff JM, Ogunremi B, Colley S, Sanghera P, Hartley A. The role of positron emission tomography/CT imaging in head and neck cancer patients after radical chemoradiotherapy. Br J Radiol. 2012 Nov;85(1019):e1120-6.
- Ong SC, Schöder H, Lee NY, Patel SG, Carlson D, Fury M, et al. Clinical utility of 18F-FDG PET/CT in assessing the neck after concurrent chemoradiotherapy for Locoregional advanced head and neck cancer. J Nucl Med. 2008;49:532—540.
- Yao M, Smith RB, Hoffman HT, Funk GF, Lu M, Menda Y, et al. Clinical significance of postradiotherapy [18F]-fluorodeoxyglucose positron emission tomography imaging in management of head-and-neck cancer-a long-term outcome report. Int J Radiat Oncol Biol Phys. 2009 May 1;74(1):9-14.
- Zundel MT, Michel MA, Schultz CJ, Maheshwari M, Wong SJ, Campbell BH, et al. Comparison of physical examination and fluorodeoxyglucose positron emission tomography/computed tomography 4-6 months after radiotherapy to assess residual head-and-neck cancer. Int J Radiat Oncol Biol Phys. 2011 Dec 1;81(5):e825-832
- Domachevsky L, Jacene HA, Sakellis CG, Kim CK. Postradiation changes in tissues: evaluation by imaging studies with emphasis on fluorodeoxyglucose- PET/computed tomography and correlation with histopathologic findings. PET Clin. 2014 Apr;9(2):217-235.
- Wallstén E, Axelsson J, Sundström T, Riklund K, Larsson A. Subcentimeter tumor lesion delineation for high-resolution 18F-FDG PET images: optimizing correction for partial-volume effects. J Nucl Med Technol. 2013 Jun;41(2):85-91. 15. Soret M, Bacharach SL, Buvat I. Partial-volume effect in PET tumor imaging. J Nucl Med. 2007;48:932—945
- Edge SB, Byrd DR, Compton CC, et al; AJCC Cancer Staging Manual (ed 7). New York, NY, Springer, 2009.
- Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373-383
- National Electrical Manufacturers Association (NEMA). Available at http:// dicom.nema.org/ Access verified 2014 September 1.
- Wahl RL, Zasadny K, Helvie M, Hutchins GD, Weber B, Cody R. Metabolic monitoring of breast cancer chemohormonotherapy using positron emission tomography: initial evaluation. J Clin Oncol. 1993 Nov;11(11):2101-2111.
- Wahl RL, Jacene H, Kasamon Y, Lodge MA. From RECIST to PERCIST: Evolving Considerations for PET response criteria in solid tumors. J Nucl Med. 2009 May;50 Suppl 1:122S-50S.










