Publication
Article
Oncology Live®
Real-World Study Shows High Rates of Endocrinopathies Are Linked to Immune Checkpoint Inhibitors
Author(s):
Use of immune checkpoint inhibitors correlates with high incidence of endocrinopathies, especially anti–CTLA-4 therapy.
Use of immune checkpoint inhibitors (ICIs) correlates with high incidence of endocrinopathies, especially anti—CTLA-4 therapy, according to a retrospective study based on real-world data. Investigators evaluated >29 million patient records from the FDA Adverse Events Reporting System (FAERS), a database of adverse events (AEs) reported by patients, healthcare providers, and others. Records were sourced from 2014 to early 2019 and investigators identified 6260 reports of endocrine-related AEs after ICI treatment.
Table. Associations of Endocrine AEs With Single-Agent ICIs (Click to Enlarge)
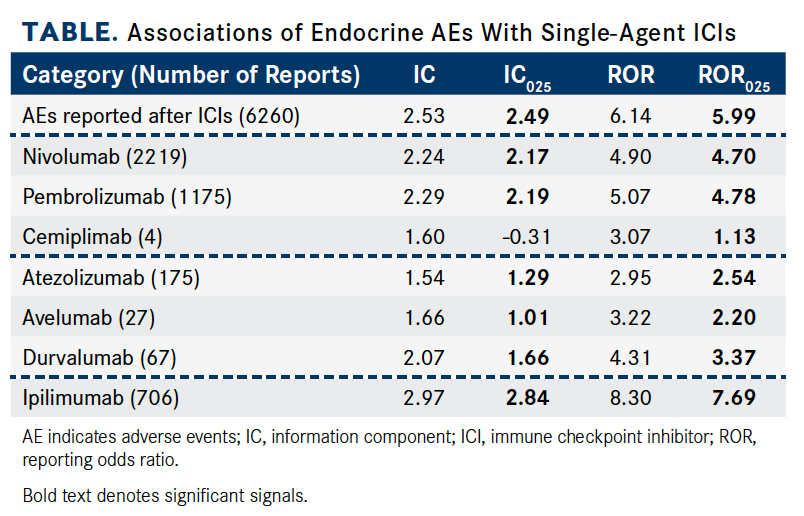
Investigators used RORs and information components (ICs) to evaluate disproportionality. The IC measures a higher- or lower-than-expected result. In the study, an ROR was defined as a signal for association if the lower limit of the 95% CI (ROR025) was >1, with ≥3 cases. The lower end of the 95% CI of IC (IC025) >0 was considered statistically significant.
Overall, patients treated with ICIs were much more likely to have reported endocrinopathies (IC025 = 2.49; ROR025 = 5.99). In addition to being the most common type of immune-related AE seen with ICIs, endocrinopathies are potentially life-threatening and irreversible.
Anti—CTLA-4 monotherapies confer a greater risk of subsequent endocrinopathies than anti- PD-1 and anti–PD-L1 monotherapies. Most endocrine-related AEs were reported with the use of anti–PD-1 therapies (n = 3398; 54.28%; IC025 = 2.20; ROR025 = 4.82). Anti—CTLA-4 agents accounted for a smaller proportion of reported endocrine-related AEs (n = 708; 11.31%) but had stronger signal values (IC025 = 2.84; ROR025 = 7.69). Notably, ipilimumab (Yervoy) had the strongest signal of ICI-associated endocrine AEs (IC025 = 2.84; ROR025 = 7.69).
The nivolumab (Opdivo) and ipilimumab combination was the most prevalent poly-ICI regimen and was used to treat 1664 patients (26.58%). Patients treated with this regimen were significantly more likely to present with an endocrine- related AE (IC025 = 3.07; ROR025 = 9.11).
Few patients (n = 64; 1.02%) were treated with the triplet nivolumab, pembrolizumab (Keytruda), and ipilimumab; nevertheless, it had the highest endocrinopathy signal (IC025 = 4.00; ROR025 = 19.44) of all combination approaches.
Agents evaluated in the study also included atezolizumab (Tecentriq), avelumab (Bavencio), durvalumab (Imfinzi), and tremelimumab. Hypothyroidism (n = 885; 14.4%), adrenal insufficiency (n = 730; 11.66%), hypophysitis (n = 688; 10.99%), and hyperthyroidism (n = 472; 7.54%) were the 4 most common endocrinopathies that occurred after ICI therapy, investigators said.
Ipilimumab, whether given as a monotherapy or combined with other agents, was heavily associated with adrenal insufficiency and hypophysitis events. Also, anti—PD-1 antibodies and the nivolumab/ipilimumab regimen were strongly associated with hypothyroidism and hyperthyroidism, investigators said. Of note, adrenal insufficiency was the only endocrinopathy commonly reported across 4 combination regimens studied and was strongly associated with nivolumab-ipilimumab (IC025 = 5.50).
Across the entire database, higher frequencies of endocrine-related AEs were observed with ICI regimens.
Zhai Y, Ye X, Hu F, et al. Endocrine toxicity of immune checkpoint inhibitors: a real-world study leveraging US Food and Drug Administration adverse events reporting system. J Immunother Cancer. 2019;7(1):286. doi: 10.1186/ s40425-019-0754-2.
Anti—PD-1, anti–PD-L1, and anti–CTLA-4 monotherapies were associated with endocrine-related complications. Further, the incidence of endocrinopathies were reported more often for ICI combination therapies than ICI monotherapy (reporting odds ratio [ROR] = 2.00; 95% CI, 1.89-2.11; Table).









