Publication
Article
Oncology Live®
Targeted Cancer Therapies: Awakening the
Author(s):
As its moniker As its moniker "guardian of the genome" suggests, p53 has become recognized as one of the most important cancer-related molecules in the cell.
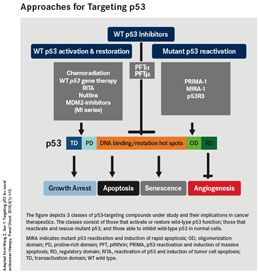
Approaches for Targeting p53. The figure depicts 3 classes of p53-targeting compounds under study and their implications in cancer therapeutics.
As its moniker "guardian of the genome" suggests, p53 has become recognized as one of the most important cancer-related molecules in the cell. As yet, p53-based research has not had a significant impact on cancer management. However, researchers continue to uncover complexities of and roles for the p53 pathway in both normal and cancerous cells, and the future for p53-targeted therapies is looking brighter than ever.
A Major Barrier to Tumorigenesis
TP53, the gene encoding the human p53 protein, was discovered through the combined efforts of a number of different researchers in the late 1970s, but it wasn't until the 1990s that its vital role as a tumor suppressor was uncovered. It was this discovery that led to its being named "Molecule of the Year" by Science magazine in 1993.
p53 is a transcription factor that, upon activation, moves into the nucleus and regulates the expression of a host of target genes. Hundreds of p53-responsive genes have been reported, with diverse biological functions, but the key function of p53 is to act as a major barrier to tumorigenesis by responding to cell stress.
Cell stress causes aberrations, such as mutations, to be introduced into the cell's DNA that, if allowed to accumulate, can eventually lead to the development of cancer. Thus, an appropriate response to cell stress is essential to maintaining the integrity of the genome and preventing cancer. Under normal conditions, p53 is present at low levels in an inactive form, kept in check principally via a protein called murine double minute 2 (MDM2), which adds ubiquitin molecules to p53, "tagging" it for destruction by the cell's degradative machinery.
Under stress conditions, for example, if DNA is damaged, p53 is activated through posttranslational modification, such as phosphorylation, and accumulates in the nucleus in an active form. Activated p53 initiates numerous cell signaling pathways that ultimately drive 1 of 2 outcomes, depending on the type of cell and extent of the damage: (1) the cell cycle is halted, meaning the cell is unable to divide and form new cells until the damage has been repaired, preventing damaged DNA from being passed on to new cells; or (2) the cell is driven to commit suicide (apoptosis).
When the Barrier Is Breached
The importance of p53 as a tumor suppressor is reflected in the fact that it is mutated in more than half of all human cancers. More than 18,000 mutations in the TP53 gene have been identified thus far. It is most commonly mutated in lung (70%), colon, head and neck, ovarian, bladder (60%), and stomach (45%) cancers. Approximately 90% of mutations are found within the DNA-binding domain, rendering p53 unable to activate target genes.
Germinal mutations in p53 are associated with the rare Li-Fraumeni syndrome, in which individuals are predisposed to a variety of cancers.
In addition to mutations in TP53, p53 function can also be disrupted through a number of other routes, including alterations in mediators of p53 function, such as MDM2, and viral inactivation of p53. In 90% of cervical cancers, p53 is inactivated by a human papillomavirus-encoded protein, E6.
2 Sides to the p53 Coin
As the vital role of p53 was uncovered, the excitement reached a crescendo, and p53 became the focus of intensive cancer research around the globe. There was much speculation about the significant potential for p53-targeted cancer therapies, yet more than 30 years later that potential has yet to be realized.
Part of the complication arises from the finding that p53 has the potential to play 2 opposing roles in cancer progression: killer and protector. The foundation of conventional treatments, such as chemotherapy and radiation therapy, rests on the premise that they induce cellular damage, which stimulates p53-dependent apoptosis, killing off tumor cells. However, p53 may also inadvertently help tumor cells to survive by activating cellular senescence (permanent cell cycle arrest) pathways instead of apoptotic pathways.
It has been reported, for example, that some types of breast cancer with wild-type p53 show resistance to chemotherapeutic regimens, and a growing number of p53-induced survival genes have been identified. It is currently unclear how p53 determines whether to induce apoptosis or senescence, but this is an area of rapidly expanding research interest.
“
Interest in p53 has driven original approaches to drug discovery that could have widespread applications. ”
Not the Usual Suspects
While p53 is an attractive drug target for cancer, based on conventional drug development processes it is not an ideal target. However, continued interest in p53 has driven a variety of original approaches to drug discovery that could have widespread applications to drug development.
In an effort to reactivate mutant or inactivated p53, new methods of screening, chemistry, and structure-based design are being used to target the structure and folding of the p53 protein, and its interactions with other proteins. Two groups of p53-targeted agents have emerged from this: (1) small molecules that bind to mutant p53 and reactivate its wild-type function; and (2) inhibitors of the interaction between p53 and its negative regulator MDM2, effectively preventing its degradation.
Among those in preclinical or early clinical development are RG7112 (Roche Group), MI-219 (Ascenta Therapeutics), JNJ-26854165 (Johnson & Johnson), reactivation of p53 and induction of tumor cell apoptosis (RITA), the carbazole derivative PhiKan083, p53 reactivation and induction of massive apoptosis (PRIMA-1) and its more potent analog mutant p53 reactivation and induction of rapid apoptosis (MIRA-1) (Aprea AB), and nutlin-3. The most advanced are PRIMA-1, JNJ-26854165, and RG7112, all currently in phase I clinical trials.
A presentation by Cellceutix Corporation at the 2011 meeting of the American Association for Cancer Research generated a substantial amount of interest when investigators reported the development of a new drug called Kevetrin, which activates p53 via a unique mechanism of action. Kevetrin outperformed current chemotherapies in a range of cancer cell lines in preclinical testing and was nongenotoxic (did not damage DNA). Cellceutix has filed a new drug application with the FDA and is planning clinical trials.
Researchers are also exploiting the genetic concept of "synthetic lethality" in searching for new p53-targeted therapies. The principle behind this is that there may be other defects that could be introduced into cancer cells that, on top of the p53 mutation, will selectively kill cancer cells, while not affecting normal cells with wild-type p53. A screen to identify compounds that inhibited the growth of p53-deficient tumor cells identified paclitaxel (an inhibitor of microtubule polymerization) and metformin (an antidiabetic drug).
Gene therapy is another approach to targeting p53 in cancer and involves the delivery of wild-type p53 into tumor cells using viral vectors. This approach was first developed in the 1990s. Gendicine (Shenzhen SiBiono GeneTech) has been approved in China since 2003. In the United States, development of Advexin (Introgen Therapeutics, Inc) stumbled in 2008 despite early promise.
Conversely, instead of targeting mutant p53, in certain situations it can be beneficial to target wild-type p53 in normal cells. Switching off p53 in normal cells could help to protect them against the toxic effects of chemotherapy and radiation therapy, allowing higher therapeutic doses to be used without harming normal cells. Several direct inhibitors of p53 have been identified, including pifithrin (PFT)-α and PFT-µ, both being evaluated in preclinical research.
An approach known as cyclotherapy is also gaining in popularity. In this case, p53 would be switched on in normal cells, with the aim of activating cellular senescence pathways and stopping normal cells (with responsive p53) from proliferating. This is then followed by the use of a second drug that targets only proliferating tumor cells (with inactive p53).
Related >>> 5 Questions on the Development of p53
Finally, p53 vaccines are also undergoing clinical trials. Preclinical trials suggested that p53 might make an excellent candidate tumor cell antigen against which to raise a vaccine, thereby allowing patients to develop a specific immune response against tumor cells. INGN-225 is currently in phase I/II trials for small cell lung carcinoma at the H. Lee Moffitt Cancer Center and Research Institute in Tampa, Florida, and has been shown to be well tolerated while inducing a p53-specific immune response that sensitized patients to chemotherapy.
Successful development of these different avenues of clinical exploration for p53 could revolutionize current cancer therapies and benefit a huge number of patients in years to come.
Jane de Lartigue, PhD, is a freelance medical writer and editor based in the United Kingdom.
Key Research
- Bai L, Zhu W-G. p53: Structure, function and therapeutic applications. J Cancer Molecules. 2006;2(4):141-153.
- Brown CJ, Lain S, Verma CS, Fersht AR, Lane DP. Awakening guardian angels: drugging the p53 pathway. Nat Rev Cancer. 2009;9(12):862-873.
- George P. p53: How crucial is its role in cancer? Int J Curr Pharm Res. 2011;3(2):19-25.
- Kumar A, Hiran T, Holden SA, Chafai-Fadela K, et al. Kevetrin%u2122, a novel small molecule, activates p53, enhances expression of p21, induces cell cycle arrest and apoptosis in a human cancer cell line [abstract]. In: Proceedings of the 102nd Annual Meeting of the American Association for Cancer Research; April 2-6, 2011; Orlando, FL. Abstract 4470.
- Lane DP, Cheok CF, Lain S. p53-based cancer therapy. Cold Spring Harb Perspect Biol. 2010;2(9):a001222.
- Mandinova A, Lee SW. The p53 pathway as a target in cancer therapeutics: obstacles and promise. Sci Transl Med. 2011;3(64):1-7.
- Suzuki K, Matsubara H. Recent advances in p53 research and cancer treatment. J Biomed Biotechnol. Published June 16, 2011. doi:10.1155/2011/978312.
- Wang Z, Sun Y. Targeting p53 for novel anticancer therapy. Transl Onc. 2010;3(1):1-12.









