Publication
Article
Oncology Live®
The Many Shades of KRAS: Investigators Seek to Exploit Heterogeneity in Mutations
Author(s):
KRAS has topped the most wanted list of therapeutic targets in oncology for decades, but it has resolutely resisted all efforts, garnering it a reputation as undruggable.
Lung cancer
KRAS has topped the most wanted list of therapeutic targets in oncology for decades, but it has resolutely resisted all efforts, garnering it a reputation as undruggable.1 That may change as a historic FDA approval for a firstin-class KRAS G12C inhibitor inches closer. In December 2020, Amgen submitted a new drug application (NDA) to the FDA for sotorasib (AMG 510) for the treatment of patients with KRAS G12C–mutated advanced non–small cell lung cancer (NSCLC).2
If approved, sotorasib would represent a significant advance for the approximately 13% of patients with NSCLC whose tumors harbor this specific mutation3,4; with an estimated 228,820 new cases of lung cancer anticipated in the United States in 2020,5 nearly 30,000 patients would be eligible for sotorasib therapy.
A second KRAS G12C inhibitor, adagrasib (MRTX849; Mirati Therapeutics, Inc) is hot on sotorasib’s heels, and promising data from the phase 1/2 KRYSTAL-1 trial (NCT03785249), for patients with NSCLC and other cancer types, were recently presented at the 2020 Molecular Targets and Cancer Therapeutics Symposium, hosted by the European Organisation for Research and Treatment of Cancer, National Cancer Institute, and American Association for Cancer Research.6,7
The trade-off with these drugs is that they do not target all KRAS mutations, and decades of intensive research have established that KRAS-mutant cancers are highly heterogeneous.1,8,9
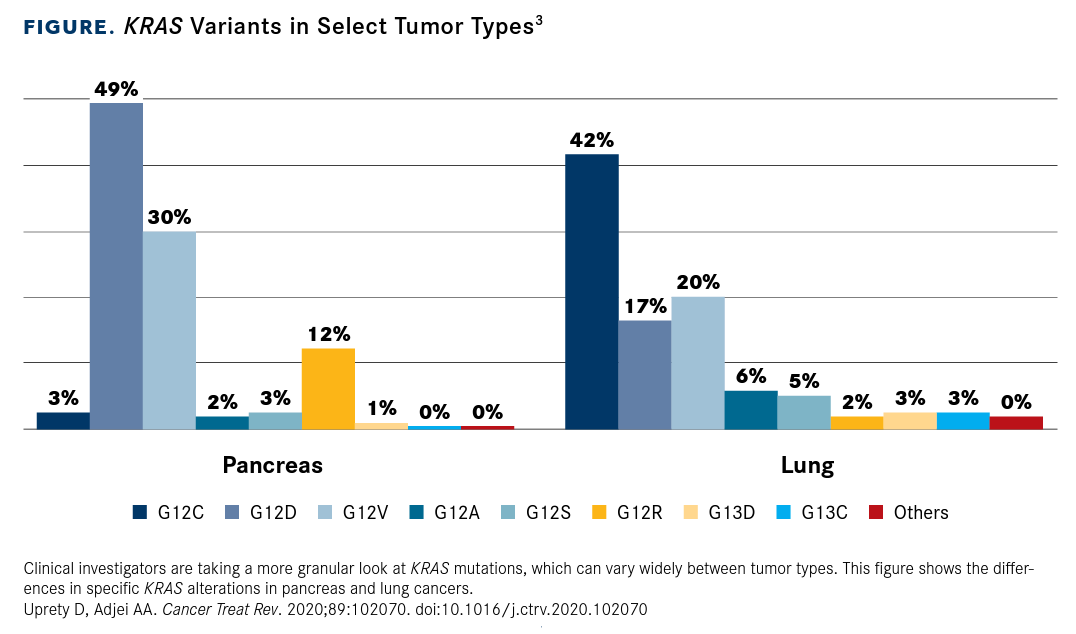
KRAS mutations are common in other cancer types as well as NSCLC, but there is substantial variation in the position and type of mutation (FIGURE3). In pancreatic ductal adenocarcinoma cases, for example, the vast majority of which harbor KRAS mutations, the most common KRAS alteration is the G12D substitution.8,10
Investigators are striving to understand the implications of these KRAS mutation patterns, with findings from some studies suggesting that they have prognostic implications and may even predict response to therapy. The picture is further complicated by the presence of common co-occurring mutations.1,3,11
The most important question is whether KRAS variants other than G12C are amenable to therapeutic targeting, and attention in the f ield is beginning to move in this direction. Strategies in clinical development include an effort to harness the power of small interfering RNA (siRNA) to inhibit the G12D mutant in pancreatic cancer by encapsulating it in a new drug delivery system, iExosomes.12,13 The G12D variant is also the focus of drug discovery efforts by Mirati, which plans to bring its lead compound, MRTX1133, to clinical trials in the next year.14
FIGURE. KRAS Variants in Select Tumor Types3
MUTATION PATTERNS VARY
KRAS proteins are encoded by RAS genes, which are among the most common genetic alterations in human cancer. These genes encode 4 highly homologous proteins— NRAS, HRAS, KRAS4A, and KRAS4B—which play a vital role in transducing extracellular signals into intracellular ones to elicit cellular responses.1
The RAS proteins cycle between an active and inactive state by binding to the small nucleotides guanosine triphosphate (GTP) and guanosine diphosphate (GDP), respectively. Upstream activation of membrane-bound growth factor receptors, such as EGFR, triggers recruitment of a guanine exchange factor to the plasma membrane, which facilitates the exchange of GDP for GTP. GTP-bound RAS undergoes a conformational change that allows it to interact with effector proteins in downstream signaling pathways, the best characterized among them being the MAPK pathway, ultimately initiating transcriptional programs in the nucleus that regulate a variety of cellular processes.3,15
RAS signaling is terminated by the binding of a GTPase-activating protein that enhances its intrinsic GTPase activity and shifts the balance back to the inactive GDP-bound state. Oncogenic mutations in the RAS genes lock the protein in the GTP-bound form, allowing the cancer cell to exploit the downstream overactivation of growth signaling pathways.3,15
Despite the similarity in the sequence and function of RAS genes, there is considerable variation in the frequency and distribution of RAS mutations across cancer types. KRAS is by far the most commonly mutated of the 3 genes (~85% of cancer cases), but NRAS mutations (~11% overall) are enriched in melanoma. HRAS mutations, despite having the lowest prevalence overall (~7%), are most common in cancers of the bladder, thyroid, prostate, and head and neck.16,17
KRAS is most highly mutated in pancreatic cancer (> 90% of cases), colorectal cancer (CRC; 30%-40% of cases), and NSCLC (15%20% of cases),1 but other common cancer types include ovarian and endometrial cancer. KRAS mutations can be found less frequently in a variety of other cancers.8
There are several hotspots within the RAS genes at which oncogenic mutations frequently occur, most commonly in codons 12, 13, 61, 117, and 146. However, the relative frequency also varies between the 3 isoforms,1,17 with codon 12 mutations the dominant form for KRAS and codon 61 for NRAS.9
The specific substitutions observed at each codon even vary across different cancer types. In NSCLC, KRAS G12C mutations (with a glycine-to-cysteine substitution) are the most common, whereas in pancreatic cancer and CRC, the G12D (glycine to aspartate) substitution is more prevalent. CRC is notable for the diversity of KRAS mutations associated with it; mutations at K117 and A146, for example, are rare overall but are found with a significant frequency in CRC.18
HOW, WHY, AND WHAT IT MEANS
Growing appreciation of the complexity of KRAS-mutant cancers leads to the inevitable questions of why such variability in mutated alleles exists, what drives it, and, above all, what the potential implications are.
At the molecular level, RAS-mutation hotspots are found around the nucleotide-binding pocket and result in enhanced GTP binding and accumulation of activated RAS. But evidence suggests that the precise mechanisms through which this occurs differ somewhat depending on the type of mutation.17,18
Codon 12 and 61 mutations affect hydrolysis, but not the rate of nucleotide exchange, whereas mutations in codons 13 and 117 affect both. Conversely, rarer codon 146 mutations affect nucleotide exchange, but not hydrolysis. Different KRAS mutants have also been shown to differentially alter the affinity of the mutant protein for downstream effectors.18 As a result, although all KRAS mutations are activating, their oncogenic potential varies, and this may impact patient prognosis and therapeutic outcomes.
The presence of KRAS mutations has generally been shown to be associated with poorer patient prognosis in NSCLC, CRC, and pancreatic cancer, but there have been conflicting reports. More recently, efforts have focused on unraveling the potential influence of individual KRAS mutation subtypes, and the results suggest that the prognostic value of a KRAS alteration may depend on which specific mutant is present and the type of cancer. For example, although codon 12 mutations are associated with decreased survival in CRC, results of some studies have shown similar outcomes for patients with codon 13 mutations and those with wild-type KRAS.3,18
The predictive value of KRAS mutations has been examined in a number of studies. Some findings indicate a negative association in specific cancer types between certain KRAS mutations and response to chemotherapy, various types of targeted therapy, and immunotherapy, but there is currently little consensus on this.3,18
The clearest role for KRAS as a predictive biomarker is in identifying patients with CRC who will not respond to EGFR-targeted therapy, but even this may be affected by the specific mutant allele. According to findings from some studies, although patients with CRC harboring most KRAS mutations are insensitive to EGFR inhibitors, KRAS G13Dmutant tumors may actually respond to these agents. This may be partly explained by the observation that the G13D mutation induces EGFR expression.18,19
The picture is further complicated by the common co-occurrence of other mutations along with KRAS, including those in STK11, KEAP1, CDKN2A/B, and TP53, which may themselves affect prognosis and response to therapy.8,20
MUTANT-SPECIFIC INHIBITORS
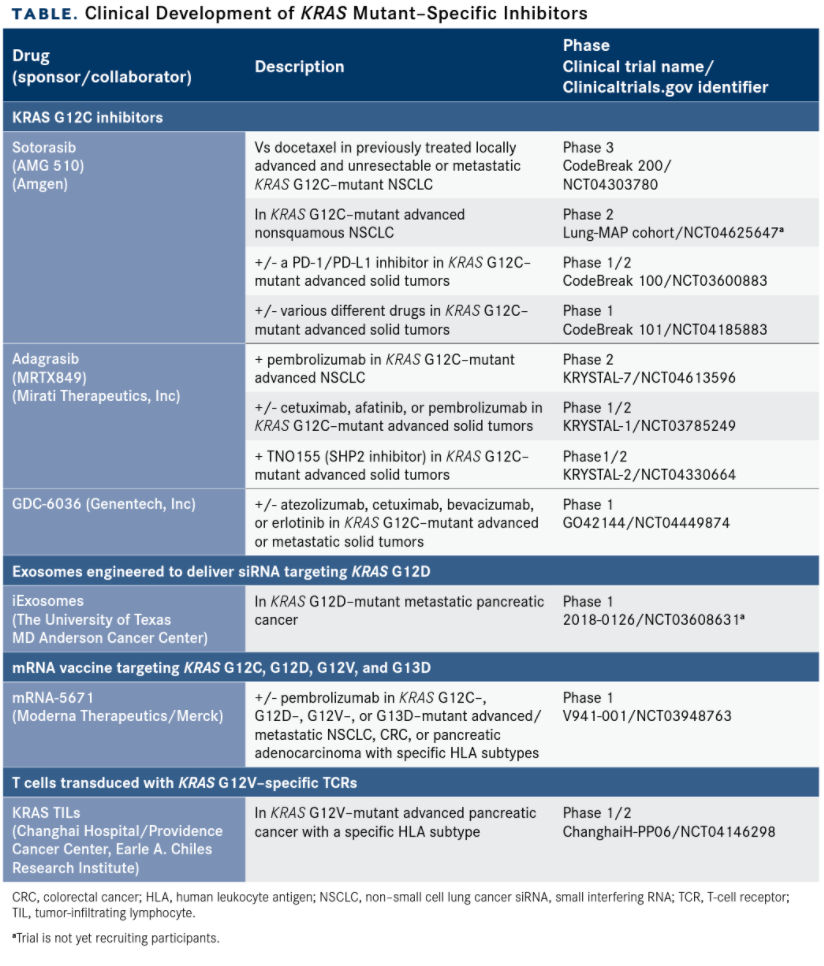
After decades of failed attempts to target the RAS pathway, which came to be thought of as undruggable, greater insight into KRAS biology has led to the discovery of potentially targetable pockets on the mutant protein.1,3,21 Signals of efficacy in several novel agents have spurred fresh industry research into KRAS-specific targets (TABLE).
Several small-molecule inhibitors of the KRAS G12C–mutant protein have been developed and represent a promising advance in the treatment of KRAS-mutant cancers. These drugs bind to a small pocket underneath the effector binding region that is only revealed in the GDP-bound form, locking the mutant protein in this inactive state.1,3
Leading the pack is sotorasib, which investigators are evaluating in clinical trials in various KRAS-mutant cancers and which has advanced the furthest in NSCLC. Amgen submitted an NDA for sotorasib for the treatment of patients with KRAS G12C–mutant advanced NSCLC, according to a company announcement in December 2020.2
The submission is supported by phase 2 results from the ongoing CodeBreak 100 study (NCT03600883) in patients with disease progression after up to 3 prior lines of therapy. Orally administered sotorasib resulted in median progression-free survival of 6.8 months (95% CI, 5.1-8.2) with a confirmed objective response rate (ORR) of 37.1% among 124 patients. The findings were presented during the International Association for the Study of Lung Cancer World Conference on Lung Cancer in January 2021. The phase 3 confirmatory CodeBreak 200 trial in NSCLC recently began enrolling patients (NCT04303780).22,23
Mirati Therapeutics has also advanced its KRAS G12C inhibitor into clinical trials. As of August 30, 2020, 108 patients with previously treated advanced solid tumors (79 NSCLC, 22 CRC, and 7 other) had been treated with adagrasib 600 mg twice daily.6,7
The ORR in patients with NSCLC was 45%, including 5 unconfirmed partial responses (PRs), and in patients with CRC it was 17%. Confirmed PRs also were observed in 1 patient with endometrial cancer and in another with pancreatic cancer. Treatmentrelated adverse events included nausea, diarrhea, vomiting, fatigue, and increased alanine aminotransferase levels.6,7
TABLE. Clinical Development of KRAS Mutant–Specific Inhibitors
BEYOND G12C
Although they undoubtedly represent a big leap forward for RAS-mutant cancers, KRAS G12C inhibitors have several key limitations that are spurring ongoing drug development efforts.
Investigators have already identified several mechanisms of resistance to KRAS G12C inhibitors. These include drug-induced selection for cells with upstream upregulation of EGFR, which maintains KRAS in the inhibitor-insensitive GTP-bound state.24 Inhibitors of the GTP-bound form are highly sought after, but their development is challenging due to the lack of potential drug binding sites.
Revolution Medicines developed its novel technology to meet this challenge. The company’s small-molecule inhibitors drive formation of a 3-protein “tri-complex” with KRAS G12C and a chaperone protein, which creates a novel binding pocket for the drug. This process also indirectly inhibits oncogenic KRAS signaling because the chaperone protein blocks KRAS G12C from interacting with downstream effectors.25
In a presentation at the RAS Targeted Drug Development Summit in September 2020, Steve Kelsey, president of research and development at Revolution Medicines, reported promising preclinical data for the company’s KRAS G12C(ON) and KRAS G12D(ON) inhibitors.26
Another limitation of KRAS G12C inhibitors is that they only target the G12C mutation; they don’t target other substitutions that are more common in other types of cancer, such as G12D, which would be a prime target for pancreatic cancer therapy.18
Mirati is developing a KRAS G12D inhibitor, MRTX1133, which has demonstrated significant tumor regression in preclinical models. The company reportedly plans to file an investigational NDA with the FDA in the f irst half of 2021.14
RNA interference (RNAi) also is a promising method for regulating the expression of target genes, particularly those that are not amenable to targeting by conventional drugs, such as KRAS. However, effective delivery of siRNA (a type of RNAi molecule) to the cancer site is challenging.27
Investigators are exploring the use of exosomes—small, fluid-filled, membranebound sacs that bud off from the cell surface and play a physiological role in transporting waste or cellular cargo—as a novel drug delivery system. iExosomes are exosomes that have been engineered to act as vehicles for siRNA targeting a gene of interest.
They are similar to liposomes and other nanoparticle drug delivery systems in development, but they express the cell-surface protein CD47, which generates the so-called “don’t eat me” signal upon receptor binding. This allows them to avoid phagocytosis by immune cells, which should extend their half-life in the circulation. Preclinical development of iExosomes targeted KRAS G12D–mutant pancreatic cancer, and a phase 1 trial (NCT03608631) is underway.12,13
Also in clinical trials are several agents based on immunotherapeutic strategies targeting mutant KRAS proteins. Merck and Moderna are developing mRNA-5671, a cancer vaccine designed to target the KRAS G12C, G12D, G12V, and G13D mutants. Meanwhile, the use of adoptive cell therapy is also being explored, with genetically engineered T-cell receptors that recognize the G12V and G12D mutants transduced into tumor-infiltrating lymphocytes and peripheral blood lymphocytes, respectively.28
References
- Liu P, Wang Y, Li X. Targeting the untargetable KRAS in cancer therapy. Acta Pharmaceutica Sinica B. 2019;9(5):871-879. doi:10.1016/j.apsb.2019.03.002
- Amgen submits sotorasib new drug application to U.S. FDA for advanced or metastatic non-small cell lung cancer with KRAS G12C mutation. News release Amgen. December 16, 2020. Accessed December 17, 2020. https://www.amgen.com/newsroom/press-releases/2020/12/amgen-submits-sotorasib-new-drug-application-to-u-s--fda-for-advanced-or-metastatic-non-small-cell-lung-cancer-with-kras-g12c-mutation
- Uprety D, Adjei AA. KRAS: from undruggable to a druggable cancer target. Cancer Treat Rev. 2020;89:102070. doi:10.1016/j.ctrv.2020.102070
- Bar-Sagi D, Knelson EH, Sequist LV. A bright future for KRAS inhibitors. Nature Cancer. 2020;1(1):25-27. doi:10.1038/s43018-019-0016-8
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020. CA: A Cancer Journal for Clinicians. 2020;70(1):7-30. doi:10.3322/caac.21590
- Johnson ML, Ou SHI, Barve M, et al. KRYSTAL-1: activity and Safety of adagrasib (MRTX849) in patients with colorectal cancer (CRC) and other solid tumors harboring a KRAS G12C mutation. Eur J Cancer. 2020;138:S2. doi:10.1016/S0959-8049(20)31077-7
- Jänne PA, Rybkin II, Spira AI, et al. KRYSTAL-1: activity and safety of adagrasib (MRTX849) in advanced/ metastatic non–small-cell lung cancer (NSCLC) harboring KRAS G12C mutation. Eur J Cancer. 2020;138:S1-S2. doi:10.1016/S0959-8049(20)31076-5
- Timar J, Kashofer K. Molecular epidemiology and diagnostics of KRAS mutations in human cancer. Cancer Metastasis Rev. 2020;39(4):1029-1038. doi:10.1007/s10555-020-09915-5
- Li S, Balmain A, Counter CM. A model for RAS mutation patterns in cancers: finding the sweet spot. Nat Rev Cancer. 2018;18(12):767-777. doi:10.1038/s41568-018-0076-6
- Gillson J, Ramaswamy Y, Singh G, et al. Small molecule KRAS inhibitors: the future for targeted pancreatic cancer therapy? Cancers. 2020;12(5). doi:10.3390/cancers12051341
- Aredo JV, Padda SK, Kunder CA, et al. Impact of KRAS mutation subtype and concurrent pathogenic mutations on non-small cell lung cancer outcomes. Lung Cancer. 2019;133:144-150. doi:10.1016/j.lungcan.2019.05.015
- Bradley CA. iExosomes target the “undruggable.” Nat Rev Cancer. 2017;17(8):453-453. doi:10.1038/nrc.2017.54
- Kamerkar S, LeBleu VS, Sugimoto H, et al. Exosomes facilitate therapeutic targeting of oncogenic KRAS in pancreatic cancer. Nature. 2017;546(7659):498-503. doi:10.1038/nature22341
- Mirati Therapeutics reports investigational adagrasib (MRTX849) preliminary data demonstrating tolerability and durable anti-tumor activity as well as initial MRTX1133 preclinical data. News release. Mirati Therapeutics, Inc. October 25, 2020. Accessed December 21, 2020. https://ir.mirati.com/news-releases/news-details/2020/Mirati-Therapeutics-Reports-Investigational-Adagrasib-MRTX849-Preliminary-Data-Demonstrating-Tolerability-and-Durable-Anti-Tumor-Activity-as-well-as-Initial-MRTX1133-Preclinical-Data/default.aspx
- Simanshu DK, Nissley DV, McCormick F. RAS proteins and their regulators in human disease. Cell. 2017;170(1):17-33. doi:10.1016/j.cell.2017.06.009
- Dunnett-Kane V, Burkitt-Wright E, Blackhall FH, Malliri A, Evans DG, Lindsay CR. Germline and sporadic cancers driven by the RAS pathway: parallels and contrasts. Ann Oncol. 2020;31(7):873-883. doi:10.1016/j.annonc.2020.03.291
- Prior IA, Hood FE, Hartley JL. The frequency of Ras mutations in cancer. Cancer Res. 2020;80(14):2969-2974. doi:10.1158/0008-5472.CAN-19-3682
- Haigis KM. KRAS alleles: the devil is in the detail. Trends Cancer. 2017;3(10):686-697. doi:10.1016/j.trecan.2017.08.006
- Stolze B, Reinhart S, Bulllinger L, Fröhling S, Scholl C. Comparative analysis of KRAS codon 12, 13, 18, 61 and 117 mutations using human MCF10A isogenic cell lines. Sci Reports. 2015;5(1):8535. doi:10.1038/srep08535
- Skoulidis F, Heymach JV. Co-occurring genomic alterations in non-small-cell lung cancer biology and therapy. Nat Rev Cancer. 2019;19(9):495-509. doi:10.1038/s41568-019-0179-8
- Ostrem JM, Peters U, Sos ML, Wells JA, Shokat KM. K-Ras(G12C) inhibitors allosterically control GTP affinity and effector interactions. Nature. 2013;503(7477):548-551. doi:10.1038/nature12796
- Amgen announces positive topline phase 2 results for investigational KRAS G12C inhibitor sotorasib in advanced non-small cell lung cancer. News release. Amgen. October 5, 2020. Accessed December 17, 2020. https://www.amgen.com/newsroom/press-releases/2020/10/amgen-announces-positive-topline-phase-2-results-for-investigational-kras-g12c-inhibitor-sotorasib-in-advanced-non-small-cell-lung-cancer
- Sotorasib provides durable clinical benefit for patients with NSCLC and KRAS mutations. International Association for the Study Of Lung Cancer; January 28, 2021. Accessed February 1, 2021. https://www.eurekalert.org/pub_releases/2021-01/iaft-spd012721.php
- Xue JY, Zhao Y, Aronowitz J, et al. Rapid non-uniform adaptation to conformation-specific KRAS(G12C) inhibition. Nature. 2020;577(7790):421-425. doi:10.1038/s41586-019-1884-x
- Amendment No. 2 to form S-1. Accessed December 18, 2020. https://www.sec.gov/Archives/edgar/data/1628171/000119312520030511/d799036ds1a.htm
- Kelsey S. Approaches to Inhibiting RAS-Driven Tumors Beyond KRASG12C. Egnyte. September 16, 2020. Accessed December 18, 2020. https://revmed.egnyte.com/dl/p2TI92ul4K
- Xin Y, Huang M, Guo WW, Huang Q, Zhang L zhen, Jiang G. Nano-based delivery of RNAi in cancer therapy. Mol Cancer. 2017;16(1):134. doi:10.1186/s12943-017-0683-y
- Salgia R, Pharaon R, Mambetsariev I, Nam A, Sattler M. The improbable targeted therapy: KRAS as an emerging target in non-small cell lung cancer (NSCLC). Cell Rep Med. 2021;2(1):100186. doi:10.1016/j.xcrm.2020.100186









