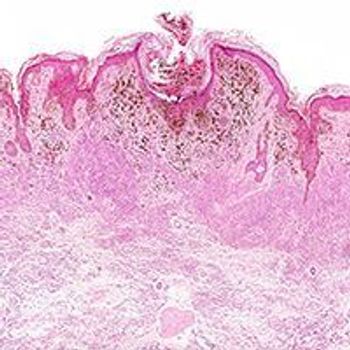
As ongoing research efforts are shifting further into personalized care, Hope S. Rugo, MD, FASCO discusses how pathologic complete response and the predictive value of residual cancer burden scoring are becoming a pivotal end point for the changing triple-negative breast cancer landscape.