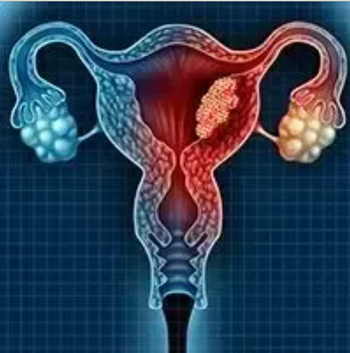
The European Medicines Agency’s Committee for Medicinal Products for Human Use has recommended the approval of pembrolizumab for use in combination with chemotherapy with or without bevacizumab in patients with persistent, recurrent, or metastatic cervical cancer who have a PD-L1 combined positive score of 1 or higher.