Hematologic Oncology
Latest News
Latest Videos

CME Content
More News

The FDA has approved a label expansion for ibrutinib with an oral suspension formulation in all current indications.

The FDA has granted priority review to the sBLA of epcoritamab for relapsed/refractory follicular lymphoma.
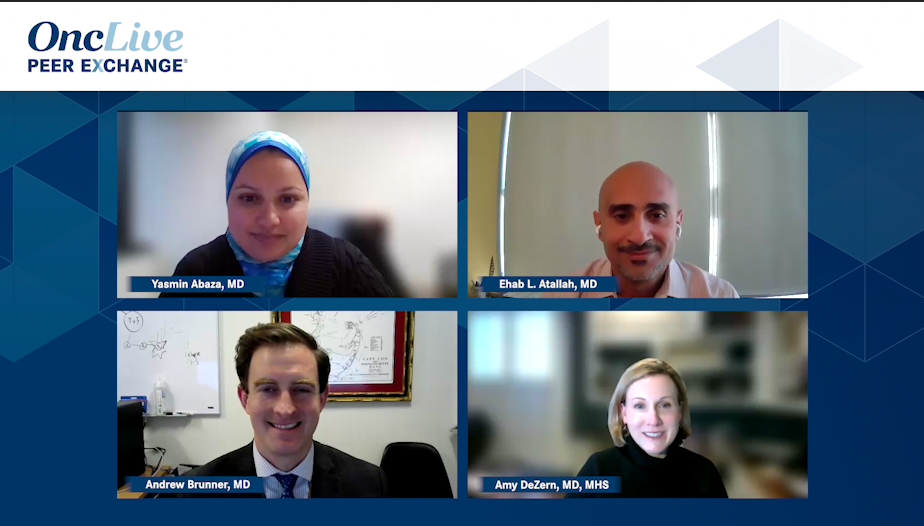
Amy DeZern, MD, MHS, discusses a retrospective study on healthcare claims for patients with MDS treated with luspatercept and reviews patient-reported outcomes from the COMMANDS trial.
Following ASH 2023, Andrew Brunner, MD, reviews key updates from the COMMANDS trial investigating frontline luspatercept in patients with lower-risk MDS.
Caron A. Jacobson, MD, and Miguel-Angel Perales, MD, look at both the regulatory and clinical components that will shape future research and utilization of CAR T-cell therapy in oncology and beyond.
Experts share tips for treating patients with VOD with defibrotide, including strategies to ensure best possible outcomes.

Real-world ruxolitinib use in chronic GVHD was primarily in the second- or third-line, lasted for a median of 8 months, and involved dose adjustments.

Encouraging data with RFS, OS, and NRM for Orca-T was also matched in a population of patients with hematologic cancers aged 55 years and older.

Jeremy L. Ramdial, MD, discusses the myeloablative conditioning regimen gemcitabine, clofarabine, and busulfan in patients with aggressive lymphomas.

In a retrospective analysis, Orca-T improved survival rates vs PTCy in patients with hematologic malignancies receiving myeloablative conditioning.

Alexandra Gomez Arteaga, MD, discusses a retrospective study of Orca-T vs post-transplant cyclophosphamide in acute leukemia and MDS.

Alice Bertaina MD, PhD, discusses T-allo10 infusion post–aβdepleted-HSCT in young patients with hematologic malignancies.
Belumosudil plus ruxolitinib elicited responses with acceptable tolerability in patients with chronic graft-vs-host disease.

Jeffery Auletta, MD, discusses the impact of post-transplant cyclophosphamide in hematologic malignancies.

Betty Hamilton, MD, discusses quality of life differences between post-transplant gilteritinib vs placebo in FLT3-ITD–positive acute myeloid leukemia.

Stephanie Lee, MD, MPH, discusses 3-year follow-up data from the phase 3 ROCKstar Study of belumosudil in cGVHD.

Treatment with immune engager therapies following relapse on ide-cel resulted in better median PFS vs other therapies for patients with multiple myeloma.

Concomitant azoles did not negatively affect efficacy with ruxolitinib or best available therapy in patients with acute graft-versus-host disease.
Ruxolitinib and belumosudil demonstrated a beneficial ORR, indicating synergistic effects in targeting multiple inflammatory pathways in chronic GVHD.

A cream formulation of ruxolitinib was deemed safe and effective compared with placebo in patients with cutaneous graft-versus-host disease.

Speculation on anticipated innovations in allo-HSCT and cellular therapy technologies in the future.

The expert panel shares emerging data on an engineered, allogeneic cell-based therapy administered as part of conditioning for allo-HSCT in patients with relapsed, refractory, or high-risk hematologic cancers.

Early data seen with acalabrutinib plus axi-cel indicate the combination’s feasibility as bridging therapy in relapsed/refractory B-cell lymphoma.

The FDA has granted IO-202 an orphan drug designation for patients with chronic myelomonocytic leukemia.
Drs Yi-Bin Chen, Corey Cutler, Hannah Choe, and Mitchell Horwitz share their predictions for the next 3 to 5 years in chronic GVHD management. The panel anticipates advancements in earlier treatment, potential elimination of frontline corticosteroids, and a shift towards preventive strategies and personalized medicine.