Myeloproliferative Neoplasms
Latest News

Latest Videos

CME Content
More News

The European Medicines Agency has validated the marketing authorization application for the use of imetelstat in the treatment of transfusion-dependent anemia in patients with lower-risk myelodysplastic syndrome.

Amy W. Zhou, MD, discusses the investigation of ruxolitinib given in conjunction with novel or emerging agents such as pacritinib and momelotinib, highlighting these agents’ potential at contributing to the treatment paradigm in myelofibrosis.
The hematologic neoplasm systemic mastocytosis is marked by the clonal proliferation of mast cells in the skin, bone marrow, gastrointestinal tract, spleen, and/or liver, caused by an activating KIT mutation in the KIT gene.

Amy W. Zhou, MD, highlights the unique mechanism of action of the JAK1/2 and ACVR1 inhibitor momelotinib and the importance of ongoing investigations of JAK inhibitor combination therapies in myelofibrosis.
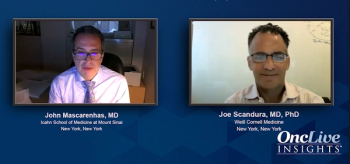
John Mascarenhas, MD, and Joe Scandura, MD, PhD, close their discussion by highlighting what to look forward to in the future of polycythemia vera treatment.

The FDA has granted fast track designation to the investigational, anti-TMPRSS6 monoclonal antibody, MWTX-003 (DISC-3405), for use in the treatment of patients with polycythemia vera, according to an announcement from Disc Medicine, Inc.

Uwe Platzbecker, MD, discusses the efficacy and safety data from the COMMANDS trial that supported the approval, and the next steps planned for luspatercept’s investigation in patients with lower-risk MDS who are transfusion independent.

Uwe Platzbecker, MD, discusses how the FDA approval of luspatercept for the treatment of anemia in patients who have not had prior treatment with erythropoiesis-stimulating agent and may require regular red blood cell transfusions could change the sequencing of agents for this patient population.

Andrew Kuykendall, MD, discusses the significance of the FDA approval of momelotinib in patients with myelofibrosis.

The FDA has approved momelotinib (Ojjaara) for the treatment of adult patients with intermediate or high-risk myelofibrosis, including primary myelofibrosis or secondary myelofibrosis, and anemia.

Key opinion leaders discuss data on the use of ruxolitinib in patients with polycythemia vera.

Joe Scandura, MD, PhD, explains how a patient with polycythemia vera and driver mutations responds to interferon treatment.

The National Institute for Health and Care Excellence has published its Final Draft Guidance recommending the use of ruxolitinib as treatment for eligible patients in England and Wales with polycythemia vera that is resistant or intolerant to hydroxycarbamide/hydroxyurea.

Following the FDA approvals of the JAK inhibitors ruxolitinib, fedratinib, and pacritinib, the treatment landscape of myelofibrosis continues to grow with the use of these agents with an additional FDA review planned for momelotinib in September 2023.

Anthony M Hunter, MD, discusses the current treatment landscape with JAK inhibitors in myelofibrosis.

Guillermo Garcia-Manero, MD, discusses the key efficacy findings from the phase 3 COMMANDS trial evaluating luspatercept-aamt in patients with myelodysplastic syndrome who were erythropoiesis-stimulating agent naïve and red blood cell transfusion dependent.

A retrospective analysis of the Surveillance, Epidemiology, and End Results database emphasized the potential impact of specific socio-racial factors, such as race, sex, and age, on survival outcomes in patients with primary myelofibrosis.

Drs Joe Scandura and John Mascarenhas discuss their treatment approaches for high-risk patients with polycythemia vera.

Experts review critical trial data in the treatment of patients with polycythemia vera.

Findings from a systematic review of several observational studies reveal that increasing disparities in survival outcomes within hematologic malignancies can be primarily attributed to 5 social determinants of health: lack of access to health insurance, treatment at a non-academic facility, low income or education level, and unmarried status.

The phase 3 COMMANDS trial investigating the use of luspatercept in patients with myelodysplastic syndrome who had not received an erythropoiesis-stimulating agent and were red blood cell transfusion dependent achieved its primary end point of superiority over treatment with an erythropoiesis-stimulating agent.

A predictive system developed using data from United States and European stem cell transplant registries was prognostic of survival in patients with myelofibrosis undergoing allogeneic hematopoietic cell transplantation.
Patients with essential thrombocythemia and polycythemia vera who also had arterial hypertension experienced a higher cumulative incidence of thrombotic adverse effects compared with those without hypertension and fewer thrombotic complications following treatment with renin angiotensin system inhibitors.

Aaron T. Gerds, MD, PhD, expands on the potential role of momelotinib in the treatment of patients with myelofibrosis who present with anemia, details the data from MOMENTUM, and explains what FDA approval of momelotinib could mean for the treatment of this patient population.

Joe Scandura, MD, PhD, summarizes the toxicity profile of interferon therapy in patients with polycythemia vera.