
Patients with ovarian cancer who begin postoperative adjuvant chemotherapy within 42 days of ending neoadjuvant chemotherapy have superior survival compared to those who initiate therapy after that point.

Your AI-Trained Oncology Knowledge Connection!


Patients with ovarian cancer who begin postoperative adjuvant chemotherapy within 42 days of ending neoadjuvant chemotherapy have superior survival compared to those who initiate therapy after that point.

Brady L. Stein, MD, discusses the differences between the types of MPNs, the NCCN guideline changes, and the important role of JAK inhibitors.

George R. Simon, MD, discusses the advancements being made with immunotherapy for the treatment of NSCLC.

Frank E. Mott, MD, discusses optimal treatment selection strategies for patients with NSCLC.

Melanie Majure, MD, discusses the optimal role chemotherapy for patients with TNBC and the importance of achieving a pCR.

The risk for graft-vs-host disease is low in children aged 2 to 12 years with acute leukemia, suggesting these patients may be good candidates for reduced preventative measures.

Sameh Mikhail, MD, discusses the promise of daratumumab, as well as the potential of medical marijuana to curb the adverse events seen with potent anticancer drugs.

The phase III ERA223 trial exploring radium-223 dichloride plus abiraterone acetate in patients with asymptomatic or mildly symptomatic chemotherapy-naïve metastatic castration-resistant prostate cancer has been unblinded early.

The FDA has approved the trastuzumab (Herceptin) biosimilar MYL-1401O (Ogivri; trastuzumab-dkst), which now has has approved indications for HER2-positive patients with breast or metastatic gastric or gastroesophageal junction adenocarcinoma.

Nivolumab improved survival versus docetaxel in a predominantly Chinese population of patients with pretreated advanced or metastatic non-small cell lung cancer.

The FDA announced that it has approved the FoundationOne CDx assay. The Centers for Medicare & Medicaid Services issued a simultaneous announcement saying the next-generation sequencing-based in vitro diagnostic test will be covered by insurance.

Sunita D. Nasta, MD, compares the biology of peripheral and cutaneous T-cell lymphomas and offered advice on the optimal therapeutic approaches for either disease with novel agents.

Alexander E. Perl, MD, discusses the future of FLT3 inhibitors, current obstacles with treating patients with acute myeloid leukemia, and what pivotal trial results the community can expect to hear in the coming months.

Ipilimumab was associated with a 1-year overall survival rate of 75% in adolescent patients with stage III/IV unresectable malignant melanoma.

Physicians are missing opportunities to refer pediatric patients with hematologic malignancies to palliative care at the end of life, and those who are admitted to palliative care tend to have very advanced disease.

Petros Grivas, MD, shares insight on the value of combining immunotherapy regimens in the treatment of patients with urothelial carcinoma.

Children with B-cell acute lymphoblastic leukemia who were positive for CD200 and/or CD56 expression had significantly poorer overall survival and disease-free survival.
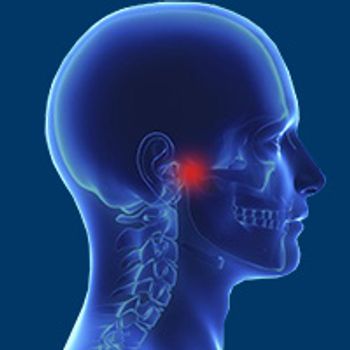
An adjusted combination of chemotherapy, radiation therapy, and surgery induced a 5-year overall survival rate of 91.3% in pediatric patients with rhabdomyosarcomas of the head and neck.

While survivors of childhood cancers are at increased risk for developing soft-tissue sarcomas compared with the general population, the absolute risk for developing STS is low except for leiomyosarcoma after retinoblastoma.

Patrick Borgen, MD, discusses the importance of neoadjuvant therapy in the treatment of patients with HER2-positive breast cancer.

The FDA has granted a priority review to a biologics license application for mogamulizumab for the treatment of patients with cutaneous T-cell lymphoma who have received at least 1 prior systemic therapy.

Avelumab did not improve overall survival compared with chemotherapy in previously treated patients with gastric or gastroesophageal junction adenocarcinoma, according to findings from the phase III JAVELIN Gastric 300 trial.

Dan T. Vogl, MD, highlights the potential with CAR T-cell therapy in the multiple myeloma paradigm.

A sNDA has been submitted to Japan’s Pharmaceuticals and Medical Devices Agency for the use of osimertinib (Tagrisso) as a frontline treatment for patients with inoperable or recurrent EGFR-positive NSCLC.

Sara M. Tolaney, MD, discusses the state of adjuvant treatment for patients with HER2-positive breast cancer.

Neeraj Agarwal, MD, recaps the latest advancements in newly diagnosed RCC.

Jacqueline Claudia Barrientos, MD, discusses the appropriate treatment strategies for elderly patients with chronic lymphocytic leukemia.

Susan R. Rheingold, MD, discusses the impact of CAR T-cell therapy on patients with ALL and options for patients who relapse on treatment.

Lajos Pusztai, MD, discusses neoadjuvant and adjuvant treatment decisions for patients with HER2-positive breast cancer, as well as the potential of immunotherapy in the metastatic setting.

Columbia University and NewYork-Presbyterian announced today that Florence Irving and her late husband, Herbert Irving, have given $700 million to the two institutions to dramatically advance research and clinical programs for the treatment of cancer.