
Gregory Roloff, MD, details data on consolidation therapy with blinatumomab or CAR T-cell therapy for B-ALL, and the role of these agents in this setting.

Your AI-Trained Oncology Knowledge Connection!


Gregory Roloff, MD, details data on consolidation therapy with blinatumomab or CAR T-cell therapy for B-ALL, and the role of these agents in this setting.
TILT-123 plus pembrolizumab was active in platinum-resistant/refractory ovarian cancer in the phase 1a PROTA trial and the phase 1b portion is ongoing.
The addition of tumor treating fields to paclitaxel did not lead to a statistically significant overall survival improvement in platinum-resistant ovarian cancer.

Jaye Gardiner, PhD, postdoctoral fellow at Fox Chase Cancer Center to receive a grant from the nonprofit Uplifting Athletes during its Young Investigators Draft.
Findings from the safety review of SON-1010 plus trabectedin in patients with advanced leiomyosarcoma or liposarcoma were positive.

The investigational new drug application for the RDC SKB107 for the management of solid tumor bone metastases has been approved in China.

The FDA has approved cabozantinib (Cabometyx) for use in select patients with pancreatic and extra-pancreatic neuroendocrine tumors.

Tess O’Meara, MD, MHS, and Kelsey H. Natsuhara, MD, discuss their presentations from the OncLive National Fellows Forum at SABCS.

Toon Van Gorp, MD, PhD, discusses the final analysis of the phase 3 MIRASOL trial of mirvetuximab soravtansine in FRα-positive, platinum-resistant ovarian cancer.

During Multiple Myeloma Awareness Month, Joshua Richter, MD, highlights symptom recognition and precursor conditions for the malignancy.
Median PFS and OS were comparable between age groups when CAR T-cell therapy was given as treatment for patients with relapsed/refractory LBCL.

Benjamin Herzberg, MD, highlights novel therapies in the KRAS-mutated NSCLC landscape and ongoing questions research is seeking to answer in this space.

Olalekan O. Oluwole, MD, discusses research findings showing that patients with LBCL may not need prolonged hospital monitoring following axi-cel infusion.

Roswell Park Comprehensive Cancer Center surgeons will share their expertise during the 2025 annual meeting of the Society of Surgical Oncology.
The development of nemvaleukin in platinum-resistant ovarian cancer will be discontinued based on interim OS data from the phase 3 ARTISTRY-7 trial.
Olvi-vec plus chemotherapy led to disease control in extensive-stage small cell lung cancer that was relapsed/refractory to platinum chemotherapy.
Nivolumab plus sunitinib displayed early-phase efficacy in advanced bone sarcoma.

Yufei Liu, MD, PhD, discusses current radiation strategies, including SBRT and IMRT, along with the benefits of radiation therapy in colorectal cancer.

Thejal Srikumar, MD, MPH, discusses the Yale School of Medicine's unique approach to offering fellows a space to address professional burnout.
A randomized trial found no significant differences in recurrence or survival with smaller radiation margins vs standard RTOG protocols in patients with HGG.

A real-world study showed OS outcomes with eribulin similar to those with trabectedin in doxorubicin-pretreated patients with advanced liposarcoma.

Benjamin Herzberg, MD, details research in KRAS G12C-mutated NSCLC with divarasib and how he currently selects between available therapies in this space.
Moffitt Cancer Center researchers found a way to boost cancer immunotherapy by targeting a protein called macrophage receptor with collagenous structure.
A Type II variation application has been submitted to the EMA for the expanded approval of sugemalimab for unresectable stage III NSCLC.
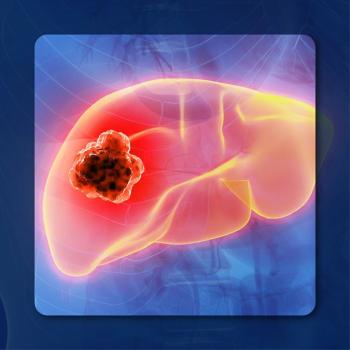
The combination of toripalimab and bevacizumab has been approved in China for the treatment of patients with unresectable or metastatic HCC.

Raajit Rampal, MD, PhD, discusses current JAK inhibitor treatment options for myelofibrosis and additional therapies being evaluated in clinical trials.

Jill Gilbert, MD, discusses professional strategy techniques that she has found useful through incredibly challenging times.
Enfortumab vedotin demonstrated a manageable safety profile and no unexpected toxicities in patients aged 80 years and older with urothelial carcinoma.

The top 5 OncLive videos of the week cover insights in gynecologic cancer, multiple myeloma, urothelial cancer, MPNs, and prostate cancer.

The risk of developing ICANS with CAR T-cell therapy was higher in patients with NHL who experienced a recent fall and could not balance on their left leg.