Hematologic Oncology
Latest News
Latest Videos

CME Content
More News

Sairah Ahmed, MD, discusses the efficacy of axicabtagene ciloleucel (axi-cel; Yescarta) in patients with refractory large B-cell lymphoma (LBCL) who achieved complete remission (CR) to any prior treatment versus those who were primary refractory.

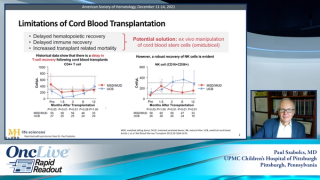
Mitchell E. Horwitz, MD, discusses the use of omidubicel versus standard myeloablative umbilical cord blood transplant in patients with hematologic malignancies.

February 9, 2021 - The European Commission has granted a full marketing authorization for fedratinib for the treatment of disease-related splenomegaly or symptoms in adult patients with primary myelofibrosis, post-polycythemia vera myelofibrosis, or post-essential thrombocytopenia myelofibrosis who have not received JAK inhibitors or who have received ruxolitinib.

February 9, 2021 - Patients with refractory large B-cell lymphoma have less to gain from axicabtagene ciloleucel if they have never achieved a complete response to any line of prior therapy.
February 9, 2021 - Ruxolitinib, when administered using a pediatric dosing algorithm, was found to elicit responses in patients with chronic graft-versus-host disease with acceptable safety.

Shaji K. Kumar, MD, discusses novel combinations that are being introduced to the relapsed/refractory setting in multiple myeloma, the value of minimal residual disease, and the importance of understanding potential toxicity when utilizing newer agents in the space.

February 9, 2021 - JSP191 in combination with low-dose total body irradiation and fludarabine has demonstrated efficacy and tolerability in older patients with minimal residual disease–positive acute myeloid leukemia and myelodysplastic syndrome who are undergoing nonmyeloablative allogeneic hematopoietic cell transplantation.

February 9, 2021 - The FDA has granted breakthrough therapy designations to asciminib for the treatment of adult patients with Philadelphia chromosome–positive chronic myeloid leukemia in chronic phase who received prior treatment with 2 or more TKIs, and for adult patients with Ph-positive CML in chronic phase whose tumors harbor the T315I mutation.



February 8, 2021 — Narsoplimab demonstrated clinical activity and a favorable survival benefit in patients with high-risk hematopoietic stem cell transplant-associated thrombotic microangiopathy, according to updated results of a pivotal phase 2 trial.

February 8, 2021 - Prior exposure to anti-CD19 therapy did not impact response to the CAR T-cell therapy lisocabtagene maraleucel in patients with relapsed/refractory large B-cell lymphoma.
February 8, 2021 - These findings may have implications for patients with hematologic malignancies, suggesting the development of protective memory against SARS-CoV2 and non-SARS human coronaviruses.



February 5, 2021 - Following stem cell transplant or treatment with CAR T-cell therapies, patients with hematologic malignancies and coronavirus disease 2019 tend to have favorable outcomes, especially if they are diagnosed in complete remission and further out from their cell infusion.

Jeremy S. Abramson, MD, discusses the FDA approval of lisocabtagene maraleucel in refractory large B-cell lymphoma.

February 5, 2021 - The FDA has granted an accelerated approval to umbralisib for the treatment of select patients with relapsed/refractory marginal zone lymphoma and relapsed/refractory follicular lymphoma.

February 5, 2021 - The FDA has approved lisocabtagene maraleucel for the treatment of adult patients with certain types of large B-cell lymphoma who have not responded to, or who have relapsed after, at least 2 other types of systemic treatment.

February 4, 2021 — The Israeli Ministry of Health has approved selinexor for the treatment of patients with multiple myeloma or diffuse large B-cell lymphoma.

February 4, 2021 - The China National Medical Products Administration has granted a conditional approval to gilteritinib for use in adult patients with relapsed or refractory acute myeloid leukemia with a FLT3 mutation detected by a validated test.
In a year marked by collaboration and innovation, therapeutic developments in oncology care once again dominated the novel drug approvals in 2020.