Lung Cancer
Latest News
Latest Videos

CME Content
More News

Bob T. Li, MD, PhD, MPH, discusses the limitations of the phase 3 IMpower010 trial in resected stage IB to IIIA non–small cell lung cancer.

Jacob Sands, MD, a physician at Dana-Farber Cancer Institute, as well as an instructor of medicine at Harvard Medical School, describes the last decade in lung cancer diagnostics and significant therapeutic advances. He also navigates the emerging classes of drugs that are beginning to make headway in the lung cancer pipeline.

Melissa L. Johnson, MD, discusses the promise of DS-7300 in small cell lung cancer and other advanced solid tumors.

Sandip Patel, MD, an associate professor of medical oncology at University of California San Diego, explains the optimal diagnostic strategies that can be utilized to better inform biomarker-based therapies in lung cancer.

Alexander I. Spira, MD, PhD, FACP, discusses the results of the phase 1 TROPION-PanTumor01 trial in non–small cell lung cancer.

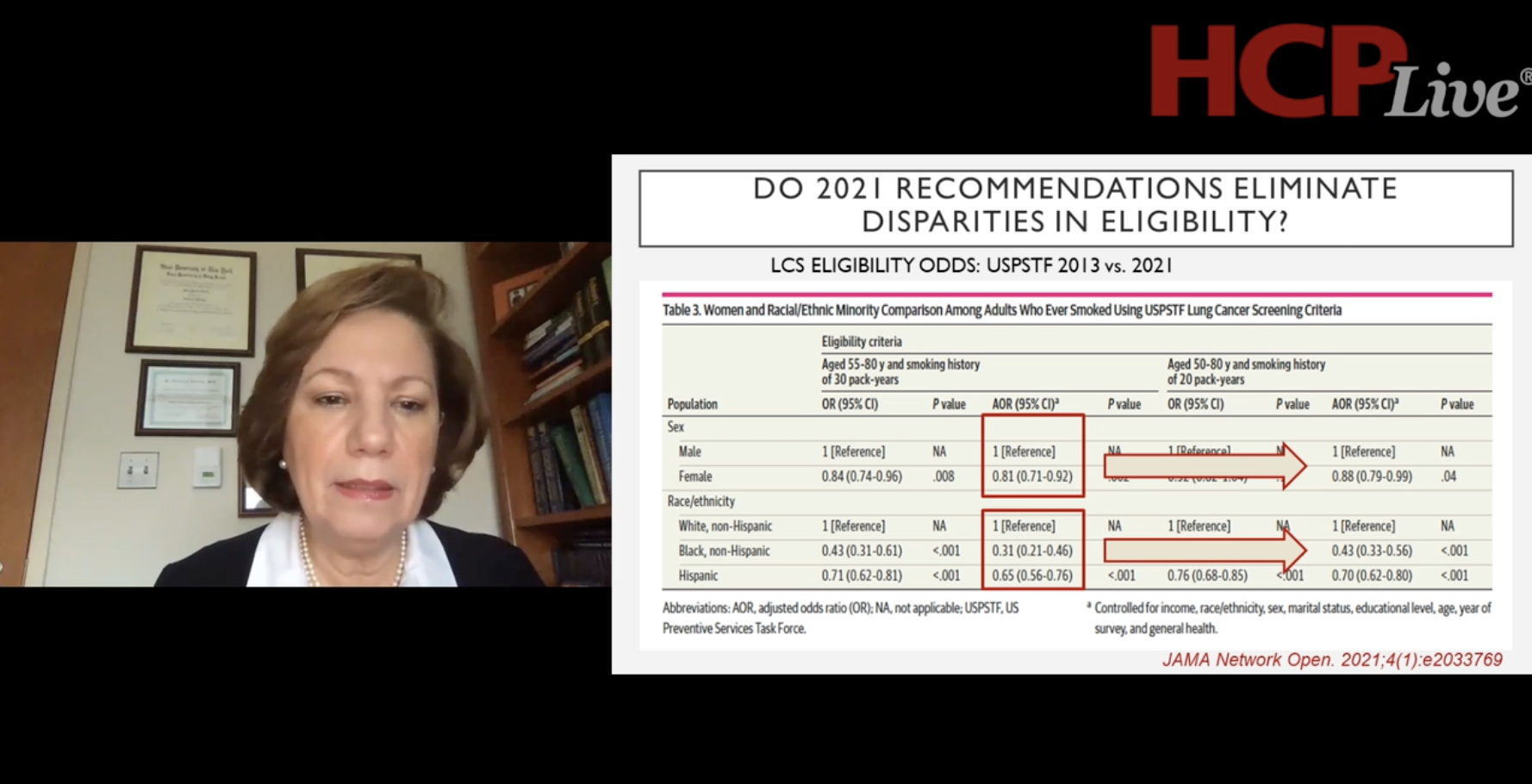
James L. Mulshine, MD, professor of internal medicine, and associate director of Institute of Translation Medicine at Rush University, discusses the benefits of establishing a timely and investigation-informed screening and referral practice for patients who are at risk for developing lung cancer.
The second part of the webinar Lung Cancer Screening and Treatment: What's Next? focuses on the review of lung cancer screening and treatment status amid the COVID-19 pandemic. Participating faculty include Albert Rizzo, MD, FCCP, FACP, FAASM; James L. Mulshine, MD; Andrea Borondy Kitts, MS, MPH; and Sandip Patel, MD.

Anish Thomas, MBBS, MD, medical oncologist, Lasker Clinical Research Scholar, Developmental Therapeutics Branch, Center for Cancer Research, National Cancer Institute, discusses the rationale for the phase 2 DDRiver SCLC 250 trial in relapsed, platinum-resistant small cell lung cancer.

Spanning metastatic non–small cell lung cancer, castration-resistant prostate cancer, and HER2-low breast cancer, here are 4 must-know clinical trials in Tennessee that community oncologists can now enroll their patients on.
Ben Levy, Mark Socinski, and Stephen Liu explain the various types of genetic alterations and protein expressions that are targetable in NSCLC and the crucial role of comprehensive genetic testing at the time of diagnosis and at the time of disease progression.

An increased understanding of driver gene alterations in advanced non–small cell lung cancer (NSCLC) has led to the development of targeted therapies that offer improved patient outcomes. Ben Levy, MD; Mark A. Socinski, MD; and Stephen Liu, MD, share their thoughts on the emerging treatment landscape and remaining unmet needs.

As novel therapies, new treatment strategies, and ongoing clinical trials continue to steer the path forward in lung cancer, treatment patterns for patients with non–small cell lung cancer, small cell lung cancer, and mesothelioma are continuing to evolve.

Aaron S. Mansfield, MD, discusses ongoing trials with chemoimmunotherapy in mesothelioma.

The addition of plinabulin to docetaxel demonstrated durable anticancer benefit in terms of overall survival when used in the second- or third-line treatment of patients with EGFR wild-type non–small cell lung cancer who had progressed on a prior platinum-based regimen.

Manish Patel, DO, discusses the role of CTLA-4 inhibitors in non–small cell lung cancer.

Nicolas Girard, MD, discusses the progression-free survival data with durvalumab observed in a real-world analysis of the phase 3 PACIFIC trial in patients with stage III non–small cell lung cancer.

The FDA has granted a breakthrough therapy designation to repotrectinib as a potential therapeutic option for patients with advanced solid tumors that harbor an NTRK gene fusion who have progressed after treatment with 1 or 2 prior TRK TKIs, with or without prior chemotherapy, and who have no satisfactory alternative options.

Amivantamab-vmjw in combination with lazertinib demonstrated significant clinical activity with durable responses in patients with EGFR-mutated non–small cell lung cancer who had progressed on osimertinib.

Anish Thomas, MD, discusses potential biomarkers of response to the combination of berzosertib plus topotecan in patients with relapsed, platinum-resistant small cell lung cancer.

Dr. Patel discusses the role of biomarker testing in lung cancer, the FDA approvals of amivantamab and mobocertinib in EGFR exon 20 insertion–positive NSCLC, and future research directions in this subset of lung cancer.
Miranda Gogishvili, MD, discusses the rationale for examining cemiplimab plus platinum-doublet chemotherapy as a frontline treatment in patients with advanced NSCLC and key findings from the phase 3 EMPOWER-Lung 3 trial.

Sanjay Popat, BSc, MBBS, FRCP, PhD, discusses the clinical implications of the final results of the phase 3 ALTA-1L trial in ALK-positive non–small cell lung cancer. Scroll to 0:07 in the video for Dr. Popat's topline points on the data.

Alexander I. Spira, MD, PhD, FACP, discusses the role of frontline brigatinib in ALK-positive non–small cell lung cancer.

Although adjuvant targeted therapy options are reserved for patients with EGFR-mutated non–small cell lung cancer, molecular testing is a critical step in the treatment of all patients with metastatic disease, where multiple driver mutations are associated with FDA-approved therapeutics.
The understanding of EGFR signaling in non–small cell lung cancer continues to evolve, helping to spark the development of novel therapies for new patient populations with uncommon alterations.