
Matthew Galsky, MD, highlights updates in metastatic urothelial cancer treatment, as well as the evolution of PARP inhibitors and the optimal use of intensified triplet therapy in prostate cancer.

Your AI-Trained Oncology Knowledge Connection!


Matthew Galsky, MD, highlights updates in metastatic urothelial cancer treatment, as well as the evolution of PARP inhibitors and the optimal use of intensified triplet therapy in prostate cancer.

Kevin M. Waters, MD, PhD, discusses key findings from a study that reinforced the rationale for Claudin18.2 testing, the prevalence of this protein in gastric cancers, and how this potential therapeutic target represents the evolving role of pathology in cancer diagnosis and care.

Christian Hurtz, PhD, an assistant professor at the Fels Cancer Institute for Personalized Medicine at the Lewis Katz School of Medicine at Temple University, has been awarded a four-year $800,000 A-Award by Alex’s Lemonade Stand Foundation for Childhood Cancer.
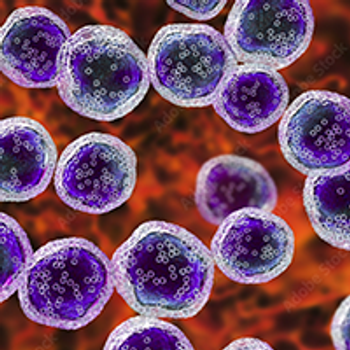
Lisocabtagene maraleucel elicited statistically significant and clinically meaningful responses in patients with relapsed or refractory follicular lymphoma and mantle cell lymphoma, meeting the primary end point of the phase 2 TRANSCEND FL and phase 1 TRANSCEND NHL 001 trials, respectively.

The FDA has granted full approval to cemiplimab-rwlc for the treatment of patients with metastatic basal cell carcinoma who previously received a hedgehog inhibitor (HHI) or for whom a HHI is not appropriate.

The FDA has granted a priority review to the supplemental biologics license application seeking to expand the current indication of luspatercept to include treatment of anemia in patients with very low- to intermediate-risk myelodysplastic syndrome who have not previously received erythropoiesis-stimulating agents and who may require red blood cell transfusions.

Treatment with pembrolizumab monotherapy resulted in stable long-term disease-free survival rates in patients with Bacillus Calmette-Guérin–unresponsive, high-risk non–muscle-invasive bladder cancer.

Treatment with TAR-200 produced complete responses and was well tolerated in patients with Bacillus Calmette-Guérin–unresponsive, high-risk non–muscle-invasive bladder cancer, according to preliminary data from the phase 2b SunRISe-1 trial.

The FDA has approved xT CDx, a 648-gene next-generation sequencing assay for solid tumor profiling, and companion diagnostic for patients with colorectal cancer.

Nivolumab following radical surgery continued to display a benefit in disease-free survival in patients with muscle-invasive urothelial carcinoma and muscle-invasive bladder cancer.
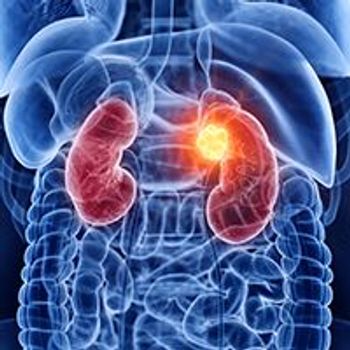
Treatment with sintilimab in combination with axitinib resulted in tumor shrinkage with tolerable adverse effects in patients with advanced fumarate hydratase–deficient renal cell carcinoma.

Specific comorbidities were linked with complications following partial nephrectomy, compared with radical nephrectomy, in patients with T1b to T2 renal cell carcinoma.

Hypertension was found to be an independent risk factor for worse survival outcomes in patients with upper tract urothelial cancer undergoing radical nephroureterectomy.

A high body mass index was linked with improved overall survival in patients with renal cell carcinoma undergoing radical nephrectomy.

Benjamin H. Lowentritt, MD, FACS, discusses prostate-specific antigen response and time to castration resistance in patients with metastatic castration-sensitive prostate cancer started on apalutamide, enzalutamide, or abiraterone acetate.

Treatment with the androgen receptor inhibitor enzalutamide plus leuprolide led to a significant reduction in the risk of metastasis or death compared with placebo plus leuprolide in patients with nonmetastatic hormone-sensitive prostate cancer with high-risk biochemical recurrence.

Patients with metastatic castration-sensitive prostate cancer treated with the androgen receptor–signaling inhibitor apalutamide achieved prostate-specific antigen responses that trended higher and rates of progression to castration resistance that trended lower than those receiving enzalutamide or abiraterone acetate/

A post-hoc analysis of the phase 3 ARASENS trial demonstrated that the addition of darolutamide to androgen deprivation therapy and docetaxel produced deep and durable prostate-specific antigen responses in patients with metastatic hormone-sensitive prostate cancer.
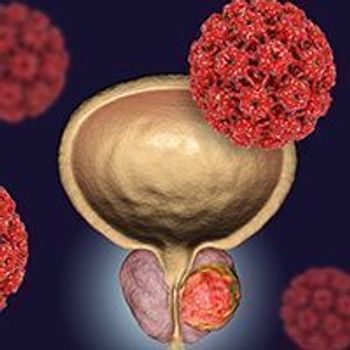
A subgroup analysis of the phase 3 ARASENS trial showed that darolutamide plus androgen deprivation therapy elicited an overall survival benefit in North American patients with metastatic hormone-sensitive prostate cancer.

Cretostimogene grenadenorepvec in combination with pembrolizumab produced high complete response rates with a tolerable safety profile in patients with Bacillus Calmette-Guérin–unresponsive non–muscle invasive bladder cancer.

Treatment with darolutamide was associated with lower rates of discontinuation and progression to metastatic disease compared with enzalutamide and apalutamide in patients with nonmetastatic castration-resistant prostate cancer.

First-line avelumab maintenance therapy prolonged survival in patients with advanced urothelial carcinoma, regardless of response to first-line chemotherapy, according to findings from an exploratory subgroup analysis of the phase 3 JAVELIN Bladder 100 trial.

The use of 68Ga-FAP-2286 PET imaging may improve treatment selection and response assessment in patients with bladder cancer, according to findings from a pilot study evaluating the diagnostic performance of this imaging technology.

In an 11-to-1 vote, the FDA’s Oncologic Drugs Advisory Committee voted that the proposed indication of olaparib in combination with abiraterone acetate and prednisone or prednisolone for the initial treatment of patients with metastatic castration-resistant prostate cancer should be restricted to those whose tumors harbor a BRCA mutation.

The addition of olaparib to abiraterone acetate resulted in numerically higher prostate-specific antigen response rates and prolonged time to PSA progression vs abiraterone alone when used in the frontline treatment of patients with metastatic castration-resistant prostate cancer.

Darolutamide given in an extended duration was linked with long-term clinical benefit and safety in patients with nonmetastatic castration-resistant prostate cancer.
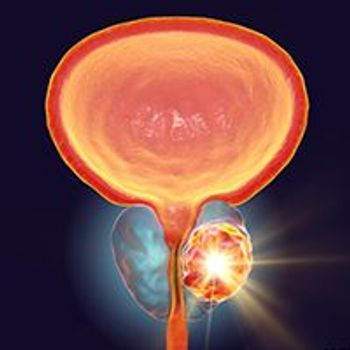
Reduced doses of apalutamide did not significantly decrease rates of skin-related adverse effects vs full-dose apalutamide in patients with advanced prostate cancer.

Treatment with lutetium 177 PSMA-I&T radioligand therapy led to prostate-specific antigen declines with an acceptable toxicity profile in patients with metastatic castration-resistant prostate cancer.

The use of zoledronic acid or other bisphosphonates was associated with a significant reduction in risk of fracture in patients with metastatic hormone-sensitive prostate cancer.

In the open-label phase 2 LITESPARK-003 study, led by Dana-Farber Cancer Institute’s Toni Choueiri, MD, researchers investigated for the first time the combination of cabozantinib, a VEGF TKI, plus belzutifan, a HIF-2α inhibitor.