
The idea to provide wearable devices to my patients first came to me 6 years ago at a charity spinning event.

Your AI-Trained Oncology Knowledge Connection!


The idea to provide wearable devices to my patients first came to me 6 years ago at a charity spinning event.

The combination of tucatinib, trastuzumab, and capecitabine continued to produce an improvement in overall survival compared with trastuzumab/capecitabine alone in patients with HER2-positive metastatic breast cancer who had stable or active brain metastases.

Mirati Therapeutics and Verastem Oncology have entered a nonexclusive clinical collaboration to evaluate the combination of adagrasib plus VS-6766 for patients with non–small cell lung cancer harboring a KRAS G12C mutation who have progressed on a KRAS G12C inhibitor in a phase 1/2 trial.

Ian E. Krop, MD, PhD, discusses highlights from 2021 in HER2-positive breast cancer and expectations for future research in 2022.

The FDA granted a breakthrough therapy designation to telisotuzumab vedotin for use in patients with advanced or metastatic EGFR wild-type, nonsquamous non–small cell lung cancer who have high levels of c-Met overexpression and whose disease has progressed on, or after, platinum-based chemotherapy.
In patients with advanced uterine leiomyosarcoma whose tumors harbor a BRCA-like phenotype, the combination of olaparib with temozolomide could represent a new standard of care.

The long-acting antibody combination of tixagevimab co-packaged with cilgavimab has been granted emergency use authorization in the United States for the pre-exposure prophylaxis of COVID-19.

Sara M. Tolaney, MD, MPH, discusses the mechanism of action that makes bispecific antibodies an intriguing approach in managing HER2-positive breast cancer, efficacy data with zanidatamab that were presented during the 2021 SABCS, and her thoughts on sequencing strategies with bispecific antibodies among other agents in the paradigm.

Early efficacy data in a phase 1 trial of the agent now known as nivolumab were not enough to convince Julie Brahmer, MD, MSc, about the potential of the investigative agent.

Driven by the anticipated launches of 5 pipeline agents and the label expansions of currently marketed drugs, theHER2-postive breast cancer market is expected to experience low growth from 2020 to 2030.

The addition of tiragolumab to atezolizumab produced a clinically meaningful improvement in progression-free survival, overall survival, and objective response rate compared with atezolizumab alone in the first-line treatment of patients with PD-L1–positive non–small cell lung cancer.

Richard Penson, MD, MBBS, discusses how he makes treatment decisions for maintenance therapy in patients with platinum-sensitive recurrent ovarian cancer.

Anil K. Sood, MD, provides insight into his approach toward the frontline maintenance treatment of women with advanced ovarian cancer.

The FDA has granted breakthrough therapy designation to CLN-081 for the treatment of patients with locally advanced or metastatic non–small cell lung cancer harboring EGFR exon 20 insertion mutations and who have received prior platinum-based chemotherapy.

The FDA has granted a rare pediatric disease designation to IMX-110 for the treatment of rhabdomyosarcoma.

Sara Tolaney, MD, MPH, discusses the data with the oral selective estrogen receptor degrader, elacestrant, and the androgen receptor agonist, enobosarm, in estrogen receptor–positive, HER2-negative breast cancer.

Maurie Markman, MD, shares how evidence strongly supports that approaches to cancer prevention have little chance of success unless those being targeted are willing to listen to, and ultimately trust, the recommendations being made by members of the scientific community.

With an influx of new trials examining the use of anti-EGFR treatments in patients with advanced colorectal cancer, attention must be paid to finding the optimal window in which to give these therapies to maximize benefit.

The FDA has granted a fast track designation to a combination comprised of the immunogene therapy quaratusugene ozeplasmia and pembrolizumab for use in select patients with late-stage non–small cell lung cancer.

Marina Kremyanskaya, MD, PhD, discusses potential new combinations for treating patients with myelofibrosis and other MPNs, with a backbone of ruxolitinib.
A new drug application seeking the approval of valemetostat tosylate for use in the treatment of patients with relapsed/refractory adult T-cell leukemia/lymphoma has been submitted to the Japanese Ministry of Health, Labour, and Welfare.

MUSC Hollings Cancer Center researchers discovered a novel mechanism showing how a certain gene mutation can allow tumors to evade detection by the immune system in colorectal cancer patients.

The European Medicines Agency has validated the Type II Variation application for fam-trastuzumab deruxtecan-nxki as a treatment for adult patients with unresectable or metastatic HER2-positive breast cancer who have previously received 1 or more anti–HER2-based regimens.
The Japanese Ministry of Health, Labour, and Welfare has approved the combination of pembrolizumab plus lenvatinib for use in patients with unresectable, advanced, or recurrent endometrial carcinoma that has progressed on chemotherapy.
Bintrafusp Alfa, a novel fusion protein designed to target the transforming growth factor beta pathway, racked up 3 clinical trial disappointments in less than a year, leaving the future of its development in question.

Adam M. Brufsky, MD, PhD, FACP, discusses the rationale to evaluate enobosarm in estrogen receptor–positive, androgen receptor–positive metastatic breast cancer, prior data observed with the agent, and the unmet needs positive results of the ARTEST trial could potentially fulfill.
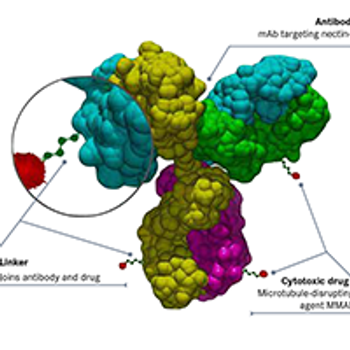
Most ongoing clinical trials exploring nectin-4 as a target involve studies of enfortumab vedotin and its potential synergy with immune checkpoint inhibitors in bladder cancer, while several other early-phase studies are testing novel agents in solid tumors.

Robert Ramirez, DO, FACP, details his path to clinical thoracic oncology after originally planning on entering the academic field early in his career.

Despite advances in treatments for T-cell acute lymphoblastic leukemia, patients have an extremely poor prognosis, highlighting the need to explore the genetic components that lead to the formation of the disease, as well as the need to discover new targeted therapeutic approaches and treatment resistance

Oleg Bess, MD, explains why data quality is essential to improving the United States health care system.