
From evaluating clinical outcomes to determining payment for services, measurements are important in health care.

Your AI-Trained Oncology Knowledge Connection!


From evaluating clinical outcomes to determining payment for services, measurements are important in health care.

The addition of durvalumab to gemcitabine and cisplatin demonstrated an improvement in overall survival vs gemcitabine and cisplatin plus placebo as frontline therapy in patients with advanced biliary tract cancer, regardless of primary tumor location.

The combination of botensilimab and balstilimab elicited deep objective responses with evidence of durability and encouraging tolerability in heavily pretreated patients with microsatellite stable, metastatic colorectal cancer.
Adding atezolizumab to neoadjuvant standard of care therapy for HER2-positive early breast cancer did not result in a better pathologic complete response compared with placebo, according to findings from the phase 3 IMpassion050 trial.

A second-line combination regimen comprised of RGX-202-01, FOLFIRI and bevacizumab demonstrated an encouraging efficacy signal and a favorable toxicity profile in patients with KRAS-mutant colorectal cancer.

The FDA has granted a breakthrough therapy designation to talquetamab for use as a potential therapeutic option in patients with relapsed or refractory multiple myeloma who received at least 4 prior lines of therapy, including a proteasome inhibitor, an immunomodulatory drug, and an anti-CD38 antibody.
Socazolimab produced encouraging response rates with an acceptable safety profile in patients with recurrent or metastatic cervical cancer, irrespective of PD-L1 status, according to findings from the dose-expansion portion of a phase 1 trial.

Second-line lisocabtagene maraleucel provides an earlier CAR T-cell treatment option that improves survival outcomes and produces a manageable safety profile in patients with relapsed/refractory large B-cell lymphoma, including those who are older and have comorbidities.

Allegheny Health Network’s Allegheny General and Forbes hospitals have earned distinguished three-star ratings from The Society of Thoracic Surgeons General Thoracic Surgery Database for their lung cancer resection surgery program.
The addition of orelabrutinib to sintilimab demonstrated good efficacy with a rapid time to response in patients with relapsed/refractory primary central nervous system lymphoma.

The novel and highly selective CDK9 inhibitor GFH009 demonstrated encouraging tolerability in patients with advanced, relapsed/refractory acute myeloid leukemia and lymphoma.

Risk stratification for patients with myelodysplastic syndrome have afforded those with asymptomatic or low-risk disease treatment pathways that can be navigated using a variety of patient characteristics. However, treatment algorithms for patients with higher-risk disease are limited to allogeneic transplant or treatment with hypomethylating agents.


Among men in the United States, incidence-based mortality is growing for penile cancer even though incidence remained consistent from 2000 to 2018.

The addition of the HER2-Vaxx vaccine to standard-of-care chemotherapy led to a significant improvement in overall survival vs SOC chemotherapy alone in patients with HER2-neu overexpressing advanced and metastatic gastric and gastroesophageal junction cancer.

The European Commission has granted approval to axicabtagene ciloleucel for the treatment of adult patients with relapsed/refractory follicular lymphoma after 3 or more prior lines of systemic therapy.

Teclistamab demonstrated superiority over physician’s choice of therapy for overall survival, progression-free survival, and time to next treatment in patients with relapsed/refractory multiple myeloma.

Florida Cancer Specialists & Research Institute welcomes Board-certified medical oncologist Richard McDonough, MD to the statewide practice.

Daniel DeAngelo MD, PhD, discusses the data seen with obecabtagene autoleucel as a novel treatment in the CAR T-cell arena, emphasizes unmet needs in B-cell acute lymphoblastic leukemia, and highlights important next steps in the study of obe-cel.

The FDA has placed a partial clinical hold on the phase 1/2 NUV-422-02 trial evaluating the selective small molecule CDK2/4/6 inhibitor NUV-422 in patients with solid tumors such as high-grade glioma, hormone receptor–positive advanced breast cancer, and metastatic castration-resistant prostate cancer.

The European Medicines Agency’s Committee for Medicinal Products for Human Use has recommended the approval of olaparib for the adjuvant treatment of patients with germline BRCA-mutated, HER2-negative, high-risk, early breast cancer who have received neoadjuvant or adjuvant chemotherapy.
The combination of anlotinib and sintilimab was found to provide a long-term survival benefit to patients with previously treated advanced cervical cancer.

The European Medicines Agency’s Committee for Medicinal Products for Human Use recommended the approval of trastuzumab deruxtecan monotherapy for patients with metastatic HER2-positive breast cancer who have received at least 1 prior anti–HER2-based regimen.
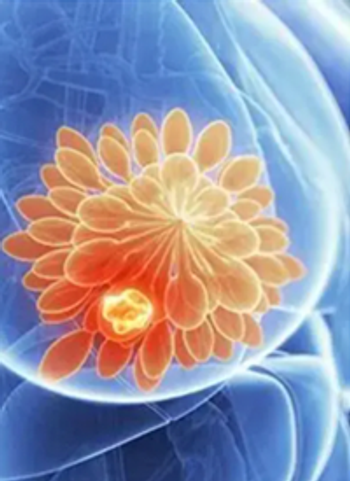
A supplemental New Drug Application has been submitted to Japan’s Ministry of Health, Labour, and Welfare seeking approval for use of trastuzumab deruxtecan for the treatment of adult patients with HER2-low unresectable or recurrent metastatic breast cancer following chemotherapy

A team of researchers with Sylvester Comprehensive Cancer Center at the University of Miami Miller School of Medicine has identified a new cellular oxygen-sensing pathway – a discovery that could potentially lead to new approaches for treating cancer and other diseases.

Adjuvant treatment with TX05 resulted in a similar disease-free survival benefit to that of trastuzumab in patients with early-stage HER2-positive breast cancer.

Batiraxcept will continue to be investigated in combination with cabozantinib, plus in combination with cabozantinib and nivolumab and as a single agent, in patients with advanced or metastatic clear cell renal cell carcinoma in a phase 2 study.

The combination of niraparib and dostarlimab produced a low overall response rate in patients with platinum-resistant ovarian cancer without a known BRCA mutation who had progressed and received prior bevacizumab, one that did not reach the threshold for second-stage accrual to the phase 2 MOONSTONE/GOG-3032 trial.

The FDA has approved lisocabtagene maraleucel as second-line therapy for adult patients with large B-cell lymphoma, including diffuse large B-cell lymphoma not otherwise specified, high-grade B-cell lymphoma, primary mediastinal large B-cell lymphoma, and follicular lymphoma grade 3B.

The combination of amivantamab-vmjw and lazertinib produced encouraging responses with acceptable safety in patients with EGFR-mutated non–small cell lung cancer who progressed on osimertinib and platinum-based chemotherapy.