Lung Cancer
Latest News

Latest Videos

CME Content
More News
Meghan J. Mooradian, MD, shares her approach to communicating with patients with NSCLC regarding treatment decisions based on molecular biomarkers.

Although emerging strategies are expanding options for treating patients with early-stage non–small cell lung cancer, stage IIIB disease continues to pose challenges. A multimodality approach may help overcome current barriers to care.

Roy S. Herbst, MD, PhD, discussed the data that supported the FDA approval of adjuvant pembrolizumab in patients with non–small cell lung cancer, highlighted the implications of the approval for the paradigm, and outlined further research opportunities.

A focused discussion on clinical trial data presented at SITC 2022 elucidating the biologic activity of tumor-infiltrating lymphocyte therapies.

Amod Sarnaik, MD, and Krishna Komanduri, MD, share their clinical experience with, and practical advice on, tumor-infiltrating lymphocyte therapy for their patients with solid tumors.

In this first episode of OncChats: Traveling Through the Lung Cancer Treatment Paradigm, Aaron Franke, MD, discusses the exploration of cellular therapies in non–small cell lung cancer.

To continue making progress in lung cancer treatment, investigators must keep pushing the science in new directions even as immunotherapy becomes more available in earlier lines.

Stephen V. Liu, MD, discusses the future of MET-targeted agents in non–small cell lung cancer based on the FDA breakthrough designation for telisotuzumab vedotin.
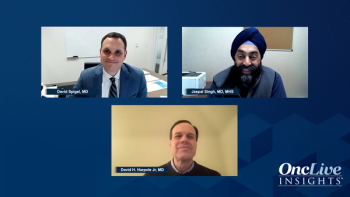
David H. Harpole Jr, MD, presents a case of a former smoker diagnosed with NSCLC, and David Spigel, MD, offers his initial impressions.

An expert panel shares perspectives on how to choose the appropriate patients with non-small cell lung cancer for lung resection.

If the FDA’s Oncologic Drugs Advisory Committee meeting held on February 10, 2022, was any indication, the days of applying foreign single country or region data to support regulatory approval are gone; instead, regulatory decisions are headed toward the need for regional consistency through multiregional clinical trials, which could help the United States overcome the persistent underrepresentation of racial and ethnic minorities in drug development.

Henry S. Park, MD, MPH, discusses key considerations when selecting an optimal treatment approach for patients with stage II and III non–small cell lung cancer.

As more agents are evaluated either in addition to or following PD-1/PD-L1 inhibitors, more information may be learned about how best to leverage alternate biomarkers, according to Marina C. Garassino, MD.

FDA approvals in recent years have started to carve out a role for immunotherapy in the frontline treatment of patients with small cell lung cancer.
Takeaways from the CheckMate 9LA, CheckMate 227, and POSEIDON trials focused on immunotherapy in non–small cell lung cancer.

Meghan J. Mooradian, MD, discusses how she makes NSCLC treatment decisions based on concurrent mutations.

Timothy Burns, MD, PhD, discusses the evolution of EGFR-targeted non–small cell lung cancer treatments, the importance of delineating small cell lung cancer subtypes, and the advantages of continued antibody-drug conjugate development across all areas of lung cancer.

Roy S. Herbst, MD, PhD, discusses the significance of the FDA approval of adjuvant pembrolizumab in patients with non–small cell lung cancer.

Shared insight on the benefits and drawbacks of using tumor-infiltrating lymphocyte therapy for treatment of solid tumors, as compared to chimeric antigen receptor (CAR) T-cell therapy.

Expert perspectives on the mechanism of action behind tumor-infiltrating lymphocyte (TIL) therapy and how it might address existing unmet needs in the treatment of solid tumors, including melanoma and non–small cell lung cancer.

Drew Moghanaki, MD, MPH, discusses questions that still need to be addressed following the phase 3 CheckMate-816 trial of the neoadjuvant combination nivolumab plus chemotherapy in patients with non–small cell lung cancer.

Michael Maris, MD, discusses key efficacy and safety data from the phase 2 OUTREACH trial in diffuse large B-cell lymphoma.

The treatment of patients with early-stage non–small cell lung cancer is rapidly evolving across the spectrum of care, particularly in the neoadjuvant setting, where evidence in favor of systemic chemoimmunotherapy regimens is growing.

Edward B. Garon, MD, MS, describes research combining angiogenesis and PD-1/PD-L1 inhibition stemming from the phase 3 IMpower150 trial in patients with metastatic nonsquamous NSCLC.

A panel of distinguished experts discuss how detection of actionable mutations affect their treatment choices in non-small cell lung cancer.