
Mohammed M. Milhem, MBBS, discusses the unique elements of oncolytic vaccines in oncology and ongoing research with RP1 in melanoma and other solid tumors.

Your AI-Trained Oncology Knowledge Connection!


Mohammed M. Milhem, MBBS, discusses the unique elements of oncolytic vaccines in oncology and ongoing research with RP1 in melanoma and other solid tumors.

Joanne Mortimer, MD, discusses current applications for HER2-directed antibody-drug conjugates, CDK4/6 inhibitors, PARP inhibitors, and checkpoint inhibitors in early-stage and metastatic breast cancer.

Dan Vogl, MD, MSCE, discusses the unique mechanism of action of modakafusp alfa and the updated results from an expansion cohort in patients with relapsed/refractory multiple myeloma.
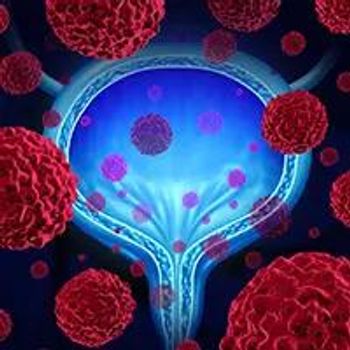
The novel non-viral gene therapy, EG-70, produced a complete response rate of 83% at 3 months in evaluable patients with high-grade non–muscle invasive bladder cancer with carcinoma in situ who were unresponsive to Bacillus Calmette-Guérin.

Saad Z. Usmani, MD, MBA, FACP, shares key takeaways from presentations given during the meeting on the evolving paradigms of frontline, early relapsed, and late relapsed multiple myeloma, the current role of bispecific antibodies and CAR T-cell therapy, and emerging immunotherapies and also trials in progress in the space.

Laura Finn, MD, provides a deep dive into pivotal data with combinations and novel agents that have changed the paradigm for patients with newly diagnosed, early relapsed, and late relapsed multiple myeloma.

John T. Cole, MD, discusses how updates in HER2-positive breast cancer have affected sequencing strategies, selecting between CDK4/6 inhibitors in the adjuvant and metastatic settings of hormone receptor HR–positive breast cancer, and the need for additional therapies in triple-negative breast cancer.
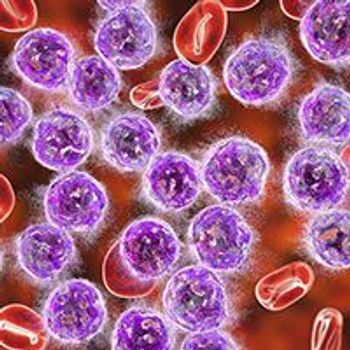
The combination of ibrutinib plus rituximab generated a high rate of complete response and undetectable minimal residual disease when used in the frontline treatment of patients with indolent clinical forms of mantle cell lymphoma, allowing for treatment interruption in most responders.

A team led by Lluis Morey, PhD, has received a five-year, $1.7 million grant from the National Institute of General Medical Sciences to investigate treatment resistance in estrogen receptor positive breast cancer.

Daniel G. Haller, MD, says he has relied on “cheerful serendipity” throughout his life and career.

The utilization of monoclonal antibodies such as nivolumab and Ipilimumab have become vital in shifting the treatment paradigm in gastroesophageal and gastrointestinal cancers.

Dhyan Chandra, PhD, discusses recent findings relating to low levels of cytochrome C in Black men with prostate cancer, plus plans for future research on this topic.

The long-term dramatic effects of the COVID-19 pandemic on cancer research and patient care present several challenges but also future opportunities.

The FDA’s Oncologic Drugs Advisory Committee voted against using single-country foreign data to support a biologics license application for sintilimab injection plus pemetrexed and platinum-based chemotherapy for the frontline treatment of patients with nonsquamous non–small cell lung cancer.

Induction treatment with ibrutinib and rituximab was safe and active in patients with mantle cell lymphoma aged 65 years or younger, allowing for fewer cycles of subsequent chemotherapy with rituximab plus hyperfractionated cyclophosphamide, vincristine, doxorubicin, and dexamethasone thereby reducing toxicity.

With 9 approved markers in non–small cell lung cancer and a plethora of established and emerging therapies that have been designed to target them, the need for molecular testing is more important than ever.

The organizations join forces to become one of the largest cancer research and treatment organizations in the country.
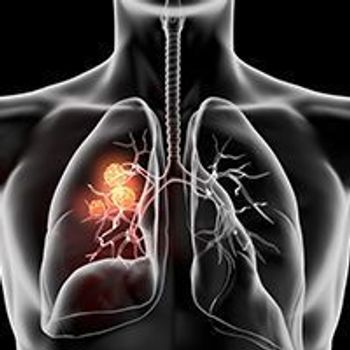
A growing recognition of the distinct clinical, pathological, and biological features of lung cancers that arise in nonsmokers is fostering greater interest in examining the molecular underpinnings of lung cancer in this patient subset.

Douglas W. Sborov, MD, MS, discusses key data and therapeutic developments that have reshaped the treatment of patients with newly diagnosed and relapsed/refractory multiple myeloma.

Jeffrey Zonder, MD, discusses his presentation at the 2021 ASH Annual Meeting on the impact of the phase 1/2 study utilizing REGN5458 in multiple myeloma.

Accelerating progress in childhood cancer faces three main obstacles: funding, drugs, and family support.

Treatment with rituximab prior to COVID-19 vaccination nearly halved the number of patients with B-cell non-Hodgkin lymphoma who developed blocking antibodies following COVID-19 vaccination compared with healthy controls.

Patients with HER2-positive, early-stage breast cancer who achieved a pathologic complete response after receiving HER2-targeted therapy experienced better outcomes in terms of disease-free survival and overall survival vs those who did not, according to data from a real-world study.

The Tisch Cancer Institute at Mount Sinai and the Samuel Waxman Cancer Research Foundation are launching a unique research program that will fund collaborations between TCI physician-scientists and colleagues from other established cancer research institutions to address the rising rates of cancer due to aging around the world.

Although cytogenetic abnormalities have helped to characterize prognosis and identify patients who are at high risk of early progression, risk stratification has little effect on management decisions for most patients with multiple myeloma.

Meredith McKean, MD, MPH, discusses how the presence of atypical BRAF mutations affects treatment selection in patients with metastatic melanoma, highlighted the ongoing KN-8701 trial, and explained why developments in this space further solidify the importance of broad molecular profiling.

Although brachytherapy is rarely utilized as a treatment for pediatric and adolescent/young adult patients in the United States, AYA patients with rhabdomyosarcoma treated with BT had favorable survival outcomes.

Lyudmila Bazhenova, MD, explores key challenges faced with diagnosing and appropriately treating patients with non–small cell lung cancer that harbors EGFR exon 20 insertion mutations and provides insight into the second-line options that have recently garnered regulatory approval.

Health care disparities remain a major issue in cancer care, and factors contributing to this inequality stretch far beyond access to clinical care.

The National Comprehensive Cancer Network is one of many organizations that is taking a stand against inequity in health care by aligning themselves with the new 3-year campaign, entitled “Close the Care Gap,” led by the Union for International Cancer Control.