Maintenance or Chemo-Free Interval in mCRC
Experts in metastatic colorectal cancer discuss when and in which patients to use a chemotherapy-free treatment interval.
Episodes in this series

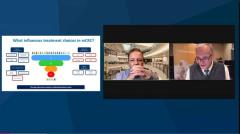
John Marshall, MD: Let’s talk about inducing the patient with unresectable cancer, too many weeds in the yard, as I like to say. But we’ve had a nice response. We’ve had some window of maintenance. This can often be a year from diagnosis now, and we start to see some progression. And I know you are very descriptive. You and I believe there’s progression, and then there’s progression. And then do you rechallenge? Do you bring in the IRI [irinotecan] if you haven’t? Do you bring your OX [oxaliplatin]. I know you probably do different things with different people but walk us through your thinking when you’re seeing that patient progressing.
Axel Grothey, MD: First of all, there is a difference between reintroduction and rechallenge, in my eyes. The reintroduction is what we do when we have induction maintenance, reintroduction strategy. For instance, it’s common with oxaliplatin if a patient’s tumor had not progressed on a regimen, on the drug, then you reintroduce it and there are tons of data of that.
Rechallenge is more likely to be used if you had tumor progression at some point and then 2 or 3 years later, you’re running out of options. You’re thinking, let’s go back to the furthest treatment away in a timely manner, and you rechallenge the cancer.
Now, when I go from first- to second-line treatment and use FOLFOX BEV [fluorouracil, oxaliplatin, bevacizumab] first line then after 8 cycles I go to a maintenance therapy. And eventually patients show tumor progression. The question of what to do depends a little on the velocity of the progression, the timing of the progression, and the adverse effects that patients might have.
There are different parameters. The patients, for instance, who had progression within 2 months of maintenance therapy, maybe had the first scan and progression-free survival. I normally switch completely and go to an irinotecan-based regimen and do something very different.
Yes, if you have maintenance therapy that works beautifully and these patients survive for a year or 2, a year and a half, no more neuropathy, and you had a great response initially, why not go back to FOLFOX? That is something. You have options, and it’s, again, not 1 size fits all.
John Marshall, MD: That first case where there’s a fairly rapid progression, where it makes them left-sided RAS wild type, are you going right? Is that when you’re going to play your EGFR [Epidermal Growth Factor Receptor]?
Axel Grothey, MD: Yes. That’s where I’ll probably put the EGFR card.
John Marshall, MD: That’s your next chance at a decent response, right?
Axel Grothey, MD: Right. Because response rate has always been higher with EGF receptor antibodies than with BEV beyond progression. It’s a different, cytostatic effect with BEV compared with a cytotoxic effect with EGF receptor antibody.
John Marshall, MD: But even if you skip second-line FOLFIRI [folinic acid, fluorouracil, irinotecan]-BEV, what it is, a 10% response rate? Whereas if you just need EGFRs in the right patient, you get up to 40% and 50%.
Axel Grothey, MD: Right. That’s actually an interesting point. We are overestimating the response rate in second line. If you look at the criteria for partial response, it’s really in the range of 10%. It’s not a lot.
John Marshall, MD: That’s important; as we all get to our third-line options, everybody says, “Well, you don’t get responses with those.” Well, frankly you don’t get that many responses in what you are enthusiastically giving in the second line, right?
Axel Grothey, MD: Right.
John Marshall, MD: That’s an important reset after that initial response, apart from a BRAF, or an EGFR, or an MSI, or HER2, if you find one, you’re not going to get a response no matter what you do. Those are the lines of therapy.
Axel Grothey, MD: Right.
John Marshall, MD: Irinotecan may be included; other biologics maybe, depending on the progression. Do you ever, and this is a bit of a provocative because I have 1 patient on this right now where I’ve given regorafenib [REGO], in what is in essence second line, because the patient doesn’t want to go to the more intensive intravenous therapy. We kind of know how to handle the drug better and it’s sort of maintenance too for me with a low-volume, good performance status patient. Have you ever done something crazy such as that?
Axel Grothey, MD: It’s not crazy. Patients tell us and guide us. It’s shared decision-making. It’s not, OK, you can push your FOLFIRI back through into the patient. You know, some patients want to have oral treatments.
And now that we have better ways to handle regorafenib in terms of toxicity management, etc, dosing strategies, it is worthwhile at least considering that. It’s not that common because, again, it’s clearly not for patients where you need a response to salvage or to get patients out of a threatening situation. Because REGO doesn’t induce responses. Same as TAS-102 [trifluridine–tipiracil], it’s the partner molecule, more or less. But it is 1 other option, and the more options we have, the better it is.
John Marshall, MD: Yes. I mean we all, for fear of not getting paid, to be blunt, we want to stick to our guidelines and our lines of therapy. And so many of these companies require us to fill out what line of therapy you’re in in order to get reimbursed. And I get that, and we don’t want to be missing expensive medicines. But at the same time, having a little bit of leeway to me is appropriate. And I believe some of these, what we call third-line therapies, in that good responding, good biology patient might in fact have some interesting…settings when patients still have excellent performance status and want rates from the unit.
Transcript Edited for Clarity