Open Questions and Future Directions in mCRC
Experts in CRC discuss questions and management of mCRC.
Episodes in this series

John Marshall, MD: I’m looking at our questions, and 1 of them is right on point of the next thought I had. If they’re HER2+ [human epidermal growth factor receptor 2], should you give EGFR therapy? There are data that that’s not a good idea.
Axel Grothey, MD: I don’t.
John Marshall, MD: Therefore, shouldn’t we know HER2 before we give EGFR therapy?
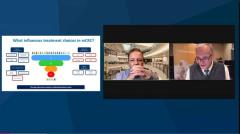
Axel Grothey, MD: Yes, and that’s 1 of the reasons why I put these 4 markers on the slides in the initial work-up. It’s RAS, BRAF, MSI [microsatellite instability], and HER2. And HER2 as a negative predictive marker; not very frequent. We talked about this 2% that’s more in the left-sided, RAS-RAF wild-type tumors that have not responded to EGF receptor antibody therapy. But the data are stronger and stronger every day that there is a negative predictive marker. And it’s a target for our treatment.
John Marshall, MD:In the metastasectomy setting, I’m leaning on it, particularly with negative adjuvant data. But I’m doing it in patients, and following them it’s a useful marker. And I do feel better, although to me it’s important to have a patient in front of you who understands what you’re talk about: that a positive is real. It means you’re in trouble almost for sure. A negative is good but is not a guarantee. We still are watching, but we feel more comfortable not treating that patient. How are you handling it?
Axel Grothey, MD: I completely agree. You know the actual real data if you have a positive result. We talk about the highest specificities, a tumor-informed test in which you profile the tumor and develop a PCR [pathologic complete response]test using the genetic fingerprint of this cancer. The predictor, the likelihood for tumor recurrence if you have a positive ctDNA, is 99.8% even if you don’t see anything on scans.
I’ve been adopting this for about a year, and there are certain patients for whom it influences treatment decision, work-up, or finding extra recurrence way before I would have done it. I would have seen it before because I do a routine scan and routine colonoscopies.
The problem I have is that there are a lot of trials ongoing or in planning. Let’s say, they’re adjuvant trials. These trials will take many years until we eventually see that they are prospectively valid and relevant. But you’re already doing it. Clinical practice and what’s happening now could actually leapfrog the evidence that we generate in prospective trials. It’s going to be an issue, but it’s a valuable tool and has influenced my treatment decisions.
John Marshall, MD: Me too. We’re going to learn a lot over the next couple of years and clean up our act in many ways with more precise decision-making.
If you got to cycle 1, day 21 of regorafenib, and you’ve worked your way up to 160 mg, and you take your week off. Then you start back at 160 mg. And I will say that I do—that’s what the study would suggest we do. But I’ve also been unhappy with that after it accumulates a little more. I find myself not infrequently going back to 120 mg during cycle 2. You don’t let your guard down into cycle 2 if you’ve escalated up to 160 mg. Watch them through cycle 2. Also liver functions—I’ve seen those go up in size. It’s a judgment call based on how that patient looks at the third week. What do you think?
Axel Grothey, MD: I completely agree. Most of my patients who are on long-term treatment are on 120 mg. There are a few who tolerate 160 mg, and there are some for whom you have to go down to 80 mg. We don’t have any data below 80 mg. It may be colon cancer. There’s some data that GIST [gastrointestinal stromal tumors] went below 80 mg. But I consider 120 mg the optimum long-term dose for my patients. The 160-mg dose can be achieved, but it’s not very frequent.
John Marshall, MD: Should we drop bolus 5-FU [5-fluorouracil], FOLFOX [5-fluorouracil, leucovorin, oxaliplatin], FOLFIRI [5-fluorouracil, leucovorin, irinotecan]? You and I might differ on this. I tend to drop it quickly. I don’t always drop it. I drop it always in FOLFOXIRI [5-fluorouracil, leucovorin, oxaliplatin, irinotecan]. Even if it’s a 45-year-old in front of me, I still drop it. But in FOLFOX [5-fluorouracil, leucovorin, oxaliplatin] and FOLFIRI [5-fluorouracil, leucovorin, irinotecan], I still hold on to my leucovorin.
Axel Grothey, MD: Leucovorin, yes.
John Marshall, MD: I’m still half-convinced, even though I taught a fellow this morning that, in theory, if I’m not giving bolus 5-FU [5-fluorouracil], I don’t need the leucovorin.
Axel Grothey, MD: That’s interesting because there are 2 studies that are old. One looked at the Roswell Park regimen and how much leucovorin we need. Because for Roswell Park, weekly bolus had 500 mg/m2 squared, and there is no difference between 20 mg and 5 mg. That’s the dose.
Then there was an EORTC [European Platform of Cancer Research] study, a randomized phase 3 study with 3 arms. One was Mayo Clinic regimen; 1 was infusion of 5-FU [5-fluorouracil] with or without leucovorin. And the progression-free survival, the primary end point, was better with leucovorin plus infusion 5-FU [5-fluorouracil] without bolus 5-FU [5-fluorouracil]. Those are phase 3 data.
I keep leucovorin around, but I don’t use bolus strategy. My goals of regimen is modified FOLFOX7 [5-fluorouracil, leucovorin, oxaliplatin]. And even with FOLFIRI [5-fluorouracil, leucovorin, irinotecan]—which I just put a patient on today—you need the bolus. It’s not confirmed by the NCCN [National Comprehensive Cancer Network] Guidelines.
Do you think FOLFOXIRI-BEV [5-fluorouracil, leucovorin, oxaliplatin, irinotecan, bevacizumab] is going to become a gold standard? As you point out, you’re still going to escalation strategy if you use FOLFOXIRI [5-fluorouracil, leucovorin, oxaliplatin, irinotecan].
Axel Grothey, MD: As much as I don’t like to think FOLFOX-BEV [5-fluorouracil, leucovorin, oxaliplatin, bevacizumab] as the 1 standard, I think FOLFOXIRI-BEV [5-fluorouracil, leucovorin, oxaliplatin, irinotecan, bevacizumab] should not be the gold standard. We have a lot of standards out there. We need to know when and how to use the tools we have.
And a triplet is indicated in some patients. I like FOLFOXIRI-BEV [5-fluorouracil, leucovorin, oxaliplatin, irinotecan, bevacizumab]. They’re good, and there are a lot more data now than there were 5 years ago. But it’s not the 1 gold standard. If I had to design a clinical trial, I would probably not use FOLFOXIRI [5-fluorouracil, leucovorin, oxaliplatin, irinotecan] as a first-line comparator. But would give it.
John Marshall, MD: On the other side is that there are still patients for who I would start with fluoropyrimidine and bevacizumab by itself.
Axel Grothey, MD: I completely agree.
John Marshall, MD: Particularly little old ladies. They do incredibly well on that regimen. Get your same 8 to 9 months of initial progression-free survival with something simple. The spectrum is in play.
We hope this discussion on OncLive® has been helpful, and we hope that what we discussed will help you in managing your patients with metastatic colorectal cancer. Thanks for taking the time. I hope everybody enjoyed the evening. Thanks to the OncLive® team who make all this possible.
Transcript Edited for Clarity