
Colorectal Cancer
Latest News
Latest Videos

CME Content
More News

Experts in CRC discuss questions and management of mCRC.
John Marshall, MD, and Axel Grothey, MD, discuss how molecular testing results guide treatment decisions in patients with mCRC.
February 3, 2021 - The Drive-By Flu-Fecal Immunochemical Test program not only provides safe and socially distanced colorectal cancer screening opportunities during the coronavirus disease 2019 pandemic, but also may help to address screening declines in diverse communities.

February 2, 2021 - ASCO has chosen molecular profiling driving progress in gastointestinal cancers as its Advance of the Year.

Kristen K. Ciombor, MD, MSCI, discusses sequencing treatment options for patients with locally advanced rectal cancer.
Experts in mCRC review data on TAS-102 and discuss how best to sequence therapies in the third or fourth line.
Axel Grothey, MD, and John Marshall, MD, discuss safety results from the ReDOS study of regorafenib in patients with mCRC.
John Marshall, MD, reviews efficacy data from the ReDOS study of regorafenib in patients with mCRC.
Experts in metastatic colorectal cancer review therapeutic options after progression for patients with mCRC.
Experts in metastatic colorectal cancer discuss when and in which patients to use a chemotherapy-free treatment interval.
Axel Grothey, MD, and John Marshall, MD, discuss strategies for optimizing first-line treatment in metastatic colorectal cancer and the role of adjuvant therapy.

January 27, 2021 - The combination of the polo-like kinase 1 inhibitor, onvansertib, with FOLFIRI and bevacizumab was found to have preliminary activity and favorable tolerability when used as a second-line treatment for patients with KRAS-mutated metastatic colorectal cancer.

Kanwal Raghav, MBBS, MD, discusses his approach to sequencing therapies for patients with resectable and unresectable colorectal cancer.

January 26, 2021 — Patients with colorectal cancer who had molecular residual disease positivity immediately after surgery were found to have a high risk of disease recurrence and longitudinal monitoring increased the predictive power of circulating tumor DNA.

January 26, 2021 - The European Commission has approved single-agent pembrolizumab for use in the frontline treatment of patients with metastatic microsatellite instability–high or mismatch repair deficient colorectal cancer.

John L. Marshall, MD, discusses recent changes to the adjuvant treatment landscape in colorectal cancer.

John H. Strickler, MD, discusses dosing strategies with regorafenib in colorectal cancer.

Nageshwara Arvind Dasari, MD, discusses the potential clinical implications with fruquintinib in metastatic colorectal cancer.

Using real-world data from more than 4000 patients with common cancers, an algorithm was able to detect variations in care that, if reduced, could result in a median savings of $26,773 per patient.

Joleen M. Hubbard, MD, discusses sequencing challenges regarding EGFR inhibitors in colorectal cancer.

Tanios S. Bekaii-Saab, MD, FACP, discusses indicators of progression in colorectal cancer.
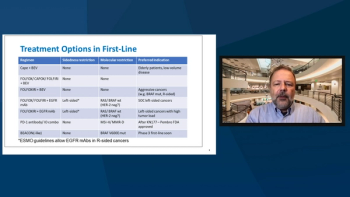
Axel Grothey, MD, and John Marshall, MD, discuss first-line treatment options in patients with metastatic colorectal cancer.

Axel Grothey, MD, discusses factors that influence treatment selection in patients with mCRC.

Daniel H. Ahn, DO, discusses the growing role of circulating tumor DNA in colorectal cancer.
Zarnie Lwin, MBBS, FRACP, discusses patient selection, including those with colorectal cancer, for the phase 2 LEAP-005 study.













































