Clinical Outcomes in HER2+ BC: FeDeriCa and PHranceSCa
Episodes in this series

Mark Pegram, MD: Joyce, can you please tell us about the safety outcomes reported in FeDeriCa? Also, because you’re the lead author on patient-reported outcomes from the PHranceSCa study at ESMO [European Society for Medical Oncology Congress], what can you tell us about the PHranceSCa study?
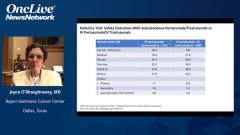
Joyce O’Shaughnessy, MD: The safety was very similar in the FeDeriCa trial with the IV [intravenous] and the subcutaneous. If you look down the list, you’d see the diarrhea, and it was very similar between the 2. With IV trastuzumab, you can get the very rare infusion reactions, systemic infusion reaction. With the subcutaneous trastuzumab-pertuzumab fixed dose, you can get a systemic injection site reaction. The occasional patient will have a systemic reaction, but it’s very similar on the IV and the subcutaneous. With regard to cardiac asymptomatic left ventricular ejection fraction declines were 2% with the IV pertuzumab-trastuzumab, and 4% with the subcutaneous trastuzumab-pertuzumab. That’s a very likely statistical similarity given the small numbers in the study. Truthfully, there really wasn’t anything else to look at in the FeDeriCa study, at least as I look at the safety data, to distinguish IV and the subcutaneous.
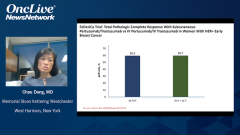
That was very, very similar to the PHranceSCa trial. Now, the PHranceSCa trial that I was involved in is a patient-reported outcome trial, and it’s also a patient choice trial. It’s quite interesting. There were 140 patients in the neoadjuvant setting who were randomized to get 3 cycles initially of IV pertuzumab and trastuzumab with chemotherapy. They had actually finished. They had finished their preoperative therapy and had surgery, and most of these patients had a pathologic complete response. They were going to be finishing the year of trastuzumab and pertuzumab. They’re postoperative and they’re randomized to either IV or subcutaneous pertuzumab and trastuzumab, so the fixed-dose combination of the subcutaneous pertuzumab-trastuzumab or standard IV pertuzumab and trastuzumab.
One group got 3 cycles of IV, then switched to 3 cycles of subcutaneous, and the other randomized group got 3 cycles of subcutaneous first and then 3 cycles of IV. The patients then said which 1 they preferred, the subcutaneous or the IV. Furthermore, they were given the choice to finish the remaining year of the trastuzumab and pertuzumab, which 1 would they like to choose? Eighty-two percent of the patients said they preferred the subcutaneous to the IV, mainly because of less time in clinic and feeling more comfortable during the actual treatment. Then 84% of patients chose to finish the year with the subcutaneous formulation. Virtually all patients said they were very satisfied or satisfied with the subcutaneous formulation.
It also was interesting that switching patients from IV to subcutaneous was also very well received by patients who cross over, and there are really no safety signals at all. If you look at the safety in PHranceSCa between the IV and the subcutaneous, there’s really no difference whatsoever. Here there was no difference in cardiac toxicity whatsoever. About a couple of percentage points of patients got a systemic IV infusion reaction with trastuzumab, and a couple of percentage points of patients got a systemic injection site reaction with the subcutaneous. But it was very minor, nothing very serious at all. That was true even if patients had been receiving IV for a while, and they switched to 3 cycles of the subcutaneous with no safety issues at all. It’s very safe to cross over from IV to subcutaneous. It was very nice. It good to see the patient’s point of view and their patient-reported outcomes, and the subcutaneous was very well received.
Mark Pegram, MD:Thank you, Joyce. Chau, can you help me understand how the safety data from PHranceSCa impacted your comfort level with subcutaneous administration with pertuzumab with trastuzumab?
Chau Dang, MD: I was very comfortable with the safety data in reviewing it and seeing that there were really no safety signals in the subcutaneous or going from IV to subcutaneous. Just in reviewing the data a note that in terms of diarrhea, which is what Joyce had mentioned earlier on, that is a toxicity we pay attention to when patients receive dual-antibody therapy. It’s in the midteens, about 16% going from IV to IV and going from IV to subcutaneous; it’s about 15%. There was no worsening rate of diarrhea. That gain of the comfort that the patients were not going to experience worse gastrointestinal toxicity. What I find striking is that when they were going from subcutaneous to subcutaneous or staying with subcutaneous, the diarrhea rate was lower, less than 10%; that was also comforting. Outside local site injection that was noted in patients getting subcutaneous, bordering around 20%, the other toxicity rates were comparable. I think the data from PHranceSCa gave me much comfort that this is a safe combination.
Transcript edited for clarity.