Efficacy of Fixed-Dose Subcutaneous Therapy for HER2+ BC
Episodes in this series

Mark Pegram, MD: I’d like to lead us into segment 2 of our session to talk in more detail about subcutaneous formulation of trastuzumab and pertuzumab, and then talk about some of the clinical data. I’d like to lead off by talking about the formulation aspects of this combination treatment by catalyzing the hydrolysis of hyaluronan, a constituent of the extracellular matrix, hyaluronidase lowers to the viscosity of hyaluronan, thereby increasing tissue permeability.
Recombinant hyaluronidase, therefore, is used in medicine in conjunction with other therapeutic proteins or biologics, like monoclonal antibodies, to speed up their dispersion and delivery from the subcutaneous space of the injection site, into the vascular space and into the systemic circulation.
Let’s talk about the FeDeriCa study, which was a phase 3 neoadjuvant-adjuvant multicenter randomized, open-label, 2-arm study of subcutaneous administration of a fixed-dose combination of trastuzumab plus pertuzumab formulated with the recombinant hyaluronidase in combination with chemotherapy.
These data were presented at San Antonio [San Antonio Breast Cancer Symposium] 2019 this past December by Antoinette Tan and colleagues. Patients were centrally confirmed to have HER2 [human epidermal growth factor receptor 2]–positive invasive breast cancer with tumors greater than 2 cm or node-positive disease with stage II to IIIC disease. Five hundred patients in total were randomized 1:1 to AC [doxorubicin, cyclophosphamide] times 4 followed by a taxane with HP [trastuzumab, pertuzumab], given either subcutaneously or intravenously.
Two chemotherapy backbone choices were dose-dense AC every 2 weeks for 4 cycles, followed by weekly paclitaxel times 12, or AC given every 3 weeks times 4 cycles, followed by docetaxel every 3 weeks times 4 cycles. The stratification variables for this randomized phase 3 study were hormone receptor status, chemotherapy backbone choice, and stage.
The dosing regimens for the fixed-dose combination, given every 3 weeks for the 2 antibodies were 1200 mg of pertuzumab subcutaneously; and 600 mg of trastuzumab subcutaneously as the loading dose; and 600 mg maintenance each. For pertuzumab given IV [intravenously] every 3 weeks was 840-mg loading dose, 420-mg maintenance; trastuzumab was an 8 mg/kg load and 6 mg/kg maintenance. This was followed by 42 weeks in the adjuvant setting of either the IV or the subcutaneous dose and schedule as noted.
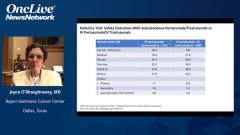
The primary objective of the study was noninferiority of the predose, cycle 8, pertuzumab serum trough concentration. A pharmacokinetic end point with the fixed-dose combination subcutaneous vs. IV pertuzumab, trastuzumab, and chemotherapy. Key secondary objectives were total pathologic complete response [pCR] in the breast and axillae and safety, including cardiotoxicity. In terms of the demographics, the patient demographics and disease characteristics were well balanced between the arms in the intent-to-treat population.
In terms of the top-line pharmacokinetic primary end point result, the predose, cycle 8 pertuzumab serum trough concentration mean was 78.5 µg/mL in the IV arm and 93.7 µg/mL in the subcutaneous arm. Nearly 93.7 is noninferior to 78.5. Thus, this study met its primary end point, which is a pharmacokinetic end point, with statistical confidence.
Dr Dang, I’ll pass it over to you to discuss the clinical outcomes in FeDeriCa.
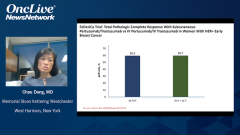
Chau Dang, MD: In terms of the clinical outcome, testing for the pathologic complete response between the 2, we saw that the pCR rates were nearly identical with subcutaneous versus IV formulation bordering on 60% with subcutaneous vs. IV. These data are very consistent with the other neoadjuvant studies, mainly TRYPHAENA and BERENICE. In TRYPHAENA study patients were receiving anthracycline followed by taxane, that’s FEC [fluorouracil, epirubicin, cyclophosphamide]–docetaxel with trastuzumab-pertuzumab, starting with anthracycline and all the way through, or FEC followed by docetaxel-trastuzumab-pertuzumab.
The third arm was the nonanthracycline arm that’s docetaxel-carboplatin-trastuzumab-pertuzumab. It was a full chemotherapy regimen with dual-antibody therapy, with a pCR rate of 60%. The FeDeriCa study had a pCR rate of 60% in both arms, which is very similar to TRYPHAENA. The BERENICE study showed similar results with a pCR of 60%, and that’s dose-dense AC followed by weekly paclitaxel with a dual-antibody therapy, or FEC followed by docetaxel and dual-antibody therapy also leading to pCR rates of about 60%.
These data are very consistent with one another, but I do want to highlight that in NeoSphere, as Joyce had mentioned, the pCR rate was about 45%. I think the reasoning why that was a little lower is because the chemotherapy and dual-antibody therapy in combination was shorter. It was 12 weeks, 3 months as opposed to the other regimens that were studied full chemotherapy of about 5 to 6 months. The take-away from the FeDeriCa study is that we saw no differences in pCR rates with full package chemotherapy with trastuzumab-pertuzumab, whether given IV or subcutaneously.
Transcript Edited for Clarity