Q&A: Practical Considerations in HER2+ Breast Cancer
Episodes in this series

Mark Pegram, MD: This brings us into our question-and-answer period. The first question I have is actually for you, Dr Dang. Given your experience as the lead author on the weekly paclitaxel plus trastuzumab-pertuzumab paper and how it compared very favorably, both in terms of efficacy as well as a far better safety profile than a docetaxel-based dual-antibody regimen in first-line metastatic HER2 [human epidermal growth factor receptor 2]–positive disease, would you consider using your regimen with the subcutaneous formulation of the dual antibodies with weekly paclitaxel?
Chau Dang, MD: I surely would. We’ve had so much experience with weekly paclitaxel and dual-antibody therapy and really saw virtually no rates of febrile neutropenia. That was a win for us, and it was well tolerated. Certainly, we would. And especially when they’re on the maintenance dual-antibody therapy, we certainly would support it. The answer is yes. We use weekly paclitaxel here much more than docetaxel, so it’s a natural chemotherapy for us.
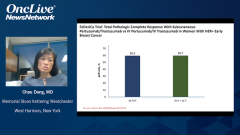
Mark Pegram, MD: Joyce, you mentioned your experience on the PHranceSCa study and the study design of all the crossover from IV [intravenous] infusion of the dual antibodies over to subcutaneous administration. Was there any difference in reactions after the crossover to the subcutaneous administration, were there any other issues to be aware of, or did any of the studies measure antidrug antibodies, etc? Is there anything else you might be concerned about, theoretically even, by the switch from IV to subcutaneous?
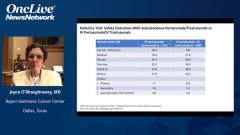
Joyce O’Shaughnessy, MD: No. Thankfully, it was really good, but you’re bringing up an important point because it’s a very practical point in the clinic for our patients who are currently on IV, whether they’re finishing their chemotherapy and going on to maintenance and subcutaneous comes up as an issue. Or they may be in the metastatic setting and you just want to switch, you want to just save the time, save the chair time. That’s why it was so carefully looked at in PHranceSCa, and there just wasn’t anything. There wasn’t any additional toxicity. There are some injection site reactions that you don’t get when you’re using a port-a-cath for your IV dual antibodies. When you do the subcutaneous into the thigh for the 8 minutes, there are some injection site reactions, but they’re grade 1/2, mostly grade 1, at about 20% or so. That would be expected whenever you give it, whether you give it right from the beginning subcutaneous or whether you’re switching. There wasn’t anything. The pharmacokinetics are really no different between the IV and the subcutaneous, so no, there are just no concerns at all that were seen in PHranceSCa.
Mark Pegram, MD: Lastly, I’d just like to find out from both of you, what are you hearing from colleagues about the pricing of this subcutaneous dual-antibody formulation compared with IV? Now we’re in the biosimilar era of trastuzumab, for instance. Do you think this will be cheaper than a biosimilar trastuzumab plus IV originator pertuzumab, for example? What are you hearing from colleagues in industry, or just a gestalt?
Chau Dang, MD: I’ll start out, Mark. I was curious myself. I went to my lead pharmacist to find out the cost is of subcutaneous trastuzumab-pertuzumab vs biosimilar trastuzumab and IV pertuzumab. The cost is very similar. If 1 is cheaper than the other, it’s by just a few dollars. It’s really very similar. I don’t like to discuss cost, but it was nice to see that the subcutaneous combination was not more expensive, and it boils down to saving time for patients and for the chemotherapy suites, that we can take care of more patients. It certainly makes sense to consider subcutaneous trastuzumab-pertuzumab over IV.
Mark Pegram, MD: There’s also the cost of the IV solution sets, all the IV tubing, and the piggyback solutions. Once you factor that in, it may very well be an advantage for the subcutaneous.
Chau Dang, MD: Right.
Mark Pegram, MD: Like you, I predicted that biosimilar trastuzumab plus originator pertuzumab would be the benchmark target for cost considerations. I’m not surprised to hear your experience from your pharmacy. Joyce, do you have anything else to add about the pricing?
Joyce O’Shaughnessy, MD: No. All told, it’s going to be a win in terms of our current savings in the health care system when you put everything together. It’s going to be a favorable thing cost-wise, and then of course that will translate into the patients’ out of pocket as well. Mainly, it’s really the time saved that is quite costly these days in the COVID-19 [coronavirus disease 2019] era and the post–COVID-19 era as well.
Mark Pegram, MD: It seems to me in other areas of medicine even, that sometimes manufacturers expect you to pay for convenience, that it might cost actually more for a more convenient drug administration. In this case, it’s actually looking like it’s coming out a little less or at least a break-even scenario. That’s gratifying and reassuring to hear that you don’t have to pay a premium for that convenience.
In closing, this has been an excellent discussion. I want to thank Dr Dang and Dr O’Shaughnessy for joining me. We hope that you found this information that we’ve presented to be valuable in your clinical practice. Thank you for watching OncLive®NewsNetwork®.
Transcript edited for clarity.