Historic Perspectives on HER2+ Breast Cancer
Episodes in this series

Mark Pegram, MD: Welcome to this OncLive® News Network® presentation. Today’s discussion will be focused on systemic therapy for early HER2 [human epidermal growth factor receptor]–positive breast cancer.Joining me today in this discussion are my esteemed colleagues: Dr Chau Dang, chief of the medical oncology service at Memorial Sloan Kettering Cancer Center’s West Harrison Outpatient Center in West Harrison, New York; and Dr Joyce O’Shaughnessy, chair of breast cancer research and the celebrating women chair in breast cancer at Baylor Scott & White Charles A. Sammons Cancer Center in Dallas, Texas.
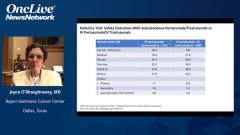
During the next 45 minutes or so we’re going to navigate through some data for a novel subcutaneous dual HER2-directed treatment and the challenges and benefits of integrating this approach into clinical practice. Our program will be divided into 3 segments: segment 1, dual HER2-targeted therapy in breast cancer; segment 2, a data review on subcutaneous trastuzumab plus pertuzumab; and segment 3, practical challenges and strategies to integrate this new information into clinical practice.
Let’s get started on our first topic. Dr Dang, I’d like you to begin with segment 1. Can you please provide a historical perspective on the treatment of HER2-positive breast cancer?
Chau Dang, MD: Thank you, Mark. Thank you so much for inviting me tonight. Over the last 2 decades I’ve seen a dramatic improvement in our patients with HER2-positive breast cancer, in both the metastatic and early stage settings. Starting with the approval of trastuzumab in 1998 in combination with chemotherapy, namely paclitaxel for the frontline treatment of patients with HER2-positive metastatic breast cancer. It was also approved as a single agent in patients who have been previously treated with chemotherapy.
For a long time, patients were given taxanes and trastuzumab, and we’ve seen a significant survival gain for about decade and a half until the publication of the APHINITY trial, which showed a significant improvement in progression-free survival and overall survival of docetaxel in combination with trastuzumab and pertuzumab over docetaxel and trastuzumab and placebo alone. This was a huge trial that led to a paradigm shift in treating our patients with metastatic HER2-positive cancer in the frontline setting.
I also want to highlight that there were other trials in between that led to the approval of several other drugs—namely apatinib, T-DM1 [trastuzumab emtansine] in the metastatic treatment of patients, and more recently, fam-trastuzumab deruxtecan and neratinib, as well at tucatinib. These other targeted therapies need to be highlighted as well.
Moving on to early stage breast cancer, in 1998, when trastuzumab was approved in the metastatic setting, 4 large studies were launched: N9831, B-31, HERA, and BCRG006 testing trastuzumab in combination with chemotherapy for the treatment of patients with high-risk early stage breast cancer. We saw that at ASCO [American Society of Clinical Oncology Annual Meeting] 2005, that the combination of trastuzumab and chemotherapy led to a significant improvement in disease-free survival and overall survival. This was the first time we saw such a significant gain in combining agents together. With the median follow-up of about 10 years now, our patients are doing very well with a median survival of 85%.
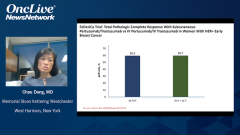
Along the way, pertuzumab was combined with trastuzumab in the adjuvant setting in the APHINITY trial in patients with high-risk HER2-positive breast cancer. We’re seeing that the combination of trastuzumab-pertuzumab-chemotherapy led to an improvement in disease-free survival, but most notably in patients with node-positive breast cancer. I also want to highlight the other key trials that are worth noting, namely the new adjuvant studies, NeoSphere, TRYPHAENA, and others testing the combination of trastuzumab-pertuzumab and chemotherapy, showing a significant improvement in pathologic complete response.
In 2013, pertuzumab-trastuzumab-chemotherapy was approved to be given to patients being treated in the neoadjuvant setting with high burden disease, namely stage II/III breast cancer with a tumor size of greater than 2 cm, or node-positive breast cancer, or inflammatory or locally advanced breast cancer. This is another indication for pertuzumab added to trastuzumab.
What’s also worth noting in the early setting is the KATHERINE study that was reported very recently showing that T-DM1, given in the adjuvant setting for patients with a residual disease after neoadjuvant treatment led to significant improvement in disease-free survival over trastuzumab. In the modern era, patients are given T-DM1after neoadjuvant treatment if they have residual disease.
Lastly, I also want to mention the neratinib story. Neratinib was also studied in the adjuvant setting from the ExteNET study, and this is a study that is also worth highlighting. This is a study where patients with high-risk breast cancer were given chemotherapy and trastuzumab. At the end of their trastuzumab treatment, they were randomized to neratinib or not. We’ve seen a significant improvement in disease-free survival in our patients, most notably in patients with hormone receptor–positive breast cancer. It’s noted here that we have a plethora of options for our patients for HER2-positive breast cancer, both in the early stage in metastatic cancer settings, and they do so well.
Transcript Edited for Clarity