The Impact of Subcutaneous Option for HER2+ BC
Episodes in this series

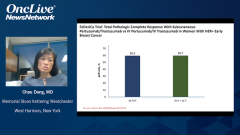
Mark Pegram, MD: I’d like to discuss the potential benefit of having a subcutaneous dual HER2-directed option for our patients. First, I’d like to discuss the label language, which stipulates that the fixed dose of trastuzumab and pertuzumab is indicated as neoadjuvant treatment of patients with HER2-positive locally advanced inflammatory or early stage breast cancer, adjuvant treatment of patients with HER2-positive early breast cancer at high risk of recurrence, and use in combination with docetaxel for treatment of patients with HER2-positive metastatic breast cancer who have not received prior anti-HER2 therapy or chemotherapy for their metastatic disease. This really illustrates how the FDA has similar label language for the indication statements for the fixed dose combination of trastuzumab and pertuzumab as the IV [intravenous] formulations. In fact, they’re identical indications. Given in totality all that we’ve talked about, the convenience, the safety, the randomized trial results, and patient-reported outcomes, when I consider what would I prefer and what I think my patients would prefer between the IV and the subcutaneous option, it boils down to an infusion time of about 2 hours, at best, if things are running on schedule in a busy infusion center, for an IV administration of these agents vs. 8 minutes for the injection. That’s, for many, a fairly easy decision because patients’ time is very valuable.
This brings us to our final segment, segment 3, which will focus on practice challenges and strategies for integrating the fixed-dose combination into clinical practice. First, I’d like to discuss the dosing schedule in a little more detail. The initial dose is 1200 mg pertuzumab and 600 mg trastuzumab, along with 30,000 units of recombinant hyaluronidase administered subcutaneously over approximately 8 minutes subcutaneous. Then every 3 weeks, this is followed by a maintenance dose of 600 mg pertuzumab, 600 mg trastuzumab, and 20,000 units of recombinant hyaluronidase over approximately a 5-minute infusion into the subcutaneous space.
This fixed-dose combination subcutaneous formulation is for use in the tops of the thighs only. The subcutaneous injection site should be alternated between left and right thighs only. New injection sites should be at least 1 inch or about 2.5 cm from the previous site on healthy skin and never into areas where the skin may be red, bruised, or tender from prior injections. Don’t split the dose between 2 syringes or between 2 sites of administration. During the treatment course with the fixed-dose monoclonal antibody combination, other medications for subcutaneous administration should preferably be injected at different sites.
Next, I’d like to call on Joyce to discuss the potential benefit of an at-home administration of this combination to patients. Is that feasible or possible, and would medical professionals still be necessary to consider this as a home treatment?
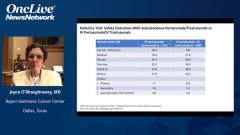
Joyce O’Shaughnessy, MD: Well, it certainly would be a change from how we run our practices, where we have our highly trained oncology nurses who are doing our infusions and our oncology pharmacists are doing the mixing. We certainly have a high comfort level with all the documentation and all the ancillary medications in case there is an infusion reaction because that same amount of safety and rigor, of course, has to take place at home. It has to be a trained nurse with experience in training specifically in this fixed-dose combination subcutaneous, and obviously a nurse experienced in treating what could be an infusion reaction or a systemic injection reaction. The medications have to be there as they are in our infusion room.
That just is going to take a little time to get the registered nurses trained, and of course the documentation and where they’re getting the medication from—that chain of custody, if you will, of that medication—and then the safety of administering it at home. Of course, it’s very safe, so that’s thankfully very good. Then of course getting the documentation back to the clinic. There is a little bit of logistical effort there, but it’s certainly nothing insurmountable. As you said, Mark, the advantages for patients is many hours. If you put in commuting times, there can be a whole day basically, a whole day away from work, away from family obligations, etc. It’s a big time-saver for patients and convenience. I think it’s worth doing. It is just a new approach, however.
Mark Pegram, MD: Finally, Dr Dang, I’d like to ask you to discuss the importance of the recent FDA approval for this fixed-dose combination subcutaneous formulation with recombinant hyaluronidase and how you might consider it along with your colleagues there at Memorial Sloan Kettering Cancer Center. Where do you think you might use this? Do you think you might actually be able to do this in a home environment? I know at our institution, we’re having a site initiation visit for a clinical trial of home administration of this formulation on a clinical trial with home visiting nurses administering the agents, etc, per protocol. Of course, they’re trained on the protocol. They have all the drugs necessary to treat anaphylactoid reactions, etc, for the subcutaneous infusion of antibodies. I’m curious to know the environment at your institution and how you think this could be impactful to your patients.
Chau Dang, MD: Sure. It is very impactful. I’ll start with the trial. We’re opening the same trial as you are, Mark, and I’m excited about it. I think in this peri–COVID-19 [coronavirus disease 2019] environment, even post–COVID-19, there are so many patients that we need to take care of, and we just don’t have enough space to take care of our patients properly. When we have a subcutaneous formulation that our patients can have at home safely, that’s the right way to go into the future, so we’re excited about this trial as well. In talking about standard of care with the fixed-dose combination, we actually had a discussion about it this past weekend.
We are certainly adopting subcutaneous delivery. As you said, the injecting is less than 10 minutes, 5 to 8 minutes. Wow, that is a time-saver for patients over the 60 minutes to 2 hours of being in the chair for that long. We would use it where it’s indicated in the neoadjuvant, adjuvant, and metastatic settings. Certainly, the first thought is if they’re on the maintenance dual-antibody therapy; that’s where it makes the most sense. If they’re on chemotherapy and they were getting intravenous trastuzumab-pertuzumab, certainly this is where we are switching over, and it’s just a mental switch because we’re so used to giving everything IV 1 after the other, and it’s more the clinician mental switch that now they can have the dual-antibody therapy in subcutaneous. We are adopting all those indications, and we’re looking forward to being able to take care of our patients in a more time-constrained setting because our clinic time is getting longer, because chemotherapy time is getting longer, because infusion times are so long. If we can cut it down, we strongly support it.
Transcript edited for clarity.