Breast Cancer
Latest News
Latest Videos

CME Content
More News

Palbociclib was not found to significantly improve invasive disease-free survival in patients with hormone receptor–positive, HER2-negative early breast cancer who had residual disease following neoadjuvant chemotherapy.

Nancy U. Lin, MD, discusses the challenges faced in the treatment of patients with HER2-positive breast cancer and brain metastases.

Howard A. “Skip” Burris, III, MD, FASCO, FACP, discusses the exploration of PARP inhibitors in triple-negative breast cancer.

Gilead Sciences’ acquisition of Immunomedics, the manufacturer of sacituzumab govitecan-hziy, is expected to advance the development of the antibody-drug conjugate not only across additional types of breast cancer beyond triple-negative disease, but also in other solid tumors.

Women with early-stage HER2-positive breast cancer who achieved a pathologic complete response to neoadjuvant HER2-directed therapy experienced prolonged event-free survival and overall survival versus those who did not.

In the metastatic breast cancer sphere, 2 chemotherapy agents have come to the forefront: capecitabine, for metastatic breast cancer, and eribulin, for triple-negative breast cancer.
Elisavet Paplomata, MD, discusses the clinical impact of tucatinib in HER2-positive breast cancer.
Treatment options for triple-negative breast cancer are historically limited; however, recent developments regarding the use of targeted therapies and various combination regimens have broadened the treatment spectrum and ushered in new clinical approaches.

Neratinib was shown to have a numerical overall survival benefit in addition to improvements in invasive disease-free survival and central nervous system recurrence in patients with HER2-positive, hormone receptor–positive, early stage breast cancer.


Sara M. Tolaney, MD, MPH, discusses the phase 2 APT and ATEMPT trials in stage I HER2-positive breast cancer.

Sara M. Tolaney, MD, MPH, discusses the evolution of treatment in HER2-positive breast cancer.

The Breast Cancer Research Foundation has awarded a 1-year, $175,000 research grant to Yale Cancer Center to study reducing re-excisions for breast conserving therapy for women following surgery for breast cancer.

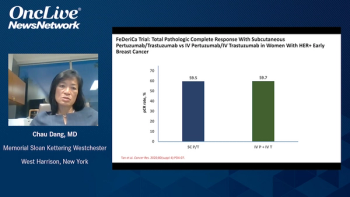
In our exclusive interview, Antoinette R. Tan, MD, of Atrium Health, discusses the FDA approval of the fixed-dose combination of pertuzumab and trastuzumab in HER2-positive breast cancer and highlights the results of the pivotal FeDeriCa trial.
Aditya Bardia, MD, MPH, discusses the need to develop additional therapies for patients with metastatic triple-negative breast cancer.
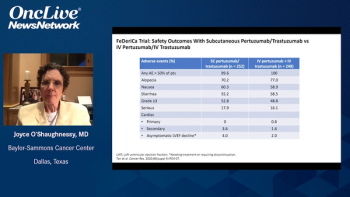

Erika P. Hamilton, MD, discusses the final overall survival results from the randomized, phase 2 nextMONARCH trial in patients with hormone receptor–positive, HER2-negative metastatic breast cancer.